Abstract
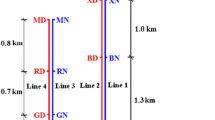
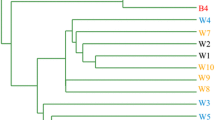
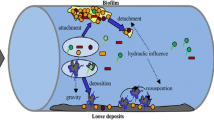
Biofilms on the inner surface of a drinking water distribution system (DWDS) affect water quality and stability. Understanding the niche differentiation of biofilm microbial communities is necessary for the efficient control of DWDS biofilms. However, biofilm studies are difficult to conduct in the actual DWDS because of inaccessibility to the pipes buried underground. Taking the opportunity of infrastructure construction and relevant pipeline replacement in China, biofilms in a DWDS (a water main and its branch pipes) were collected in situ, followed by analysis on the abundances and community structures of bacterial and archaeal using quantitative PCR and high-throughput sequencing, respectively. Results showed that archaea were detected only in the biofilms of the water main, with a range of 9.4×103~1.1×105 copies/cm2. By contrast, bacteria were detected in the biofilms of branch pipes and the distal part of the water main, with a range of 8.8×103~9.6×106 copies/cm2. Among the biofilm samples, the archaeal community in the central part of the water main showed the highest richness and diversity. Nitrosopumilus was found to be predominant (86.22%) in the biofilms of the proximal part of the water main. However, Methanobrevibacter (87.15%) predominated in the distal part of the water main. The bacterial community of the water main and branch pipes was primarily composed of Firmicutes and Proteobacteria at the phylum level, respectively. Regardless of archaea or bacteria, only few operational taxonomic units (OTUs) (<0.5% of total OTUs) were shared by all the biofilms, indicating the niche differentiation of biofilm microorganisms. Moreover, the high Mn content in the biofilms of the distal sampling location (D3) in the water main was linked to the predominance of Bacillus. Functional gene prediction revealed that the proportion of infectious disease-related genes was 0.44–0.67% in the tested biofilms. Furthermore, functional genes related to the resistance of the bacterial community to disinfections and antibiotics were detected in all the samples, that is, glutathione metabolism-relating genes (0.14–0.65%) and beta-lactam resistance gene (0.01–0.05%). The results of this study indicate the ubiquity of archaea and bacteria in the biofilms of water main and branch pipes, respectively, and pipe diameters could be a major influencing factor on bacterial community structure. In the water main, the key finding was the predominant existence of archaea, particularly Nitrosopumilus and methanogen. Hence, their routine monitoring and probable influences on water quality in pipelines with large diameter should be given more attention. Besides, since Mn-related Bacillus and suspected pathogenic Enterococcus were detected in the biofilm, supplementation of disinfectant may be a feasible strategy for inhibiting their growth and ensuring water quality. In addition, the monitoring on their abundance variation could help to determine the frequency and methods of pipeline maintenance.





Similar content being viewed by others
References
Makris KC, Andra SS, Botsaris G (2014) Pipe scales and biofilms in drinking-water distribution systems: undermining finished water quality. Crit Rev Env Sci Tec 44(13):1477–1523. https://doi.org/10.1080/10643389.2013.790746
Flemming HC, Percival SL, Walker JT (2002) Contamination potential of biofilms in water distribution systems. Water Supply 2(1):271–280. https://doi.org/10.2166/ws.2002.0032
Ginige MP, Wylie J, Plumb J (2011) Influence of biofilms on iron and manganese deposition in drinking water distribution systems. Biofouling 27(2):151–163. https://doi.org/10.1080/08927014.2010.547576
Liu G, Bakker GL, Li S et al (2014) Pyrosequencing reveals bacterial communities in unchlorinated drinking water distribution system: an integral study of bulk water, suspended solids, loose deposits, and pipe wall biofilms. Environ Sci Technol 48(10):5467–5476. https://doi.org/10.1021/es5009467
Zhou X, Zhang K, Zhang T et al (2017) An ignored and potential source of taste and odor (T&O) issues - biofilms in drinking water distribution system (DWDS). Appl Microbiol Biot 101(9):3537–3550. https://doi.org/10.1007/s00253-017-8223-7
Ding SK, Deng Y, Bond T et al (2019) Disinfection byproduct formation during drinking water treatment and distribution: a review of unintended effects of engineering agents and materials. Water Res 160:313–329. https://doi.org/10.1016/j.watres.2019.05.024
Lehtola MJ, Torvinen E, Kusnetsov J et al (2007) Survival of Mycobacterium avium, Legionella pneumophila, Escherichia coli, and Caliciviruses in drinking water-associated biofilms grown under high-shear turbulent flow. Appl Environ Microb 73(9):2854–2859. https://doi.org/10.1128/aem.02916-06
Douterelo I, Jackson M, Solomon et al (2017) Spatial and temporal analogies in microbial communities in natural drinking water biofilms. Sci Total Environ 581-582:277-288. https://doi.org/10.1016/j.scitotenv.2016.12.118
**g Z, Lu Z, Mao T et al (2021) Microbial composition and diversity of drinking water: a full scale spatial-temporal investigation of a city in northern China. Sci Total Environ 776:145986. https://doi.org/10.1016/j.scitotenv.2021.145986
Kitajima M, Cruz MC, Williams RBH et al (2021) Microbial abundance and community composition in biofilms on in-pipe sensors in a drinking water distribution system. Sci Total Environ 766:142314. https://doi.org/10.1016/j.scitotenv.2020.142314
Gomes IB, Simoes M, Simoes LC (2014) An overview on the reactors to study drinking water biofilms. Water Res 62:63–87. https://doi.org/10.1016/j.watres.2014.05.039
Zhu Z, Shan L, Zhang X et al (2021) Effects of bacterial community composition and structure in drinking water distribution systems on biofilm formation and chlorine resistance. Chemosphere 264:128410. https://doi.org/10.1016/j.chemosphere.2020.128410
Gomez-Alvarez V, Pfaller S, Pressman JG et al (2016) Resilience of microbial communities in a simulated drinking water distribution system subjected to disturbances: role of conditionally rare taxa and potential implications for antibiotic-resistant bacteria. Environ Sci-Wat Res 2:645–657. https://doi.org/10.1039/C6EW00053C
Aggarwal S, Gomez-Smith CK, Jeon Y et al (2018) Effects of chloramine and coupon material on biofilm abundance and community composition in bench-scale simulated water distribution systems and comparison with full-scale water mains. Enviro Sci Technol 52(22):13077–13088. https://doi.org/10.1021/acs.est.8b02607
Fish KE, Osborn AM, Boxall J (2016) Characterizing and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems. Environ Sci-Wat Res 2:614–630. https://doi.org/10.1039/C6EW00039H
Cerrato JM, Falkinham JO, Dieterich AM et al (2010) Manganese-oxidizing and –reducing microorganisms isolated from biofilms in chlorinated drinking water systems. Water Res 44(13):3935–3945. https://doi.org/10.1016/j.watres.2010.04.037
Kimbell LK, Lamartina EL, Kappell AD et al (2021) Cast iron drinking water pipe biofilms support diverse microbial communities containing antibiotic resistance genes, metal resistance genes, and class 1 integrons. Enviro Sci-Wat Res 7(3):584–598. https://doi.org/10.1039/d0ew01059f
Douterelo I, Sharpe RL, Boxall JB (2013) Influence of hydraulic regimes on bacterial community structure and composition in an experimental drinking water distribution system. Water Res 47:503–516. https://doi.org/10.1016/j.watres.2012.09.053
Tsagkari E, Connelly S, Liu Z et al (2022) The role of shear dynamics in biofilm formation. NPJ Biofilms Microbi 8:33. https://doi.org/10.1038/s41522-022-00300-4
Buse HY, Morris BJ, Struewing IT et al (2019) Chlorine and monochloramine disinfection of Legionella pneumophila colonizing copper and polyvinyl chloride drinking water biofilms. Appl Environ Microb 85(7):1–33. https://doi.org/10.1128/aem.02956-18
Moissl-Eichinger C, Pausan M, Taffner J et al (2018) Archaea are interactive components of complex microbiomes. Trends in Microbiology 26(1):70–85. https://doi.org/10.1016/j.tim.2017.07.004
Inkinen J, Siponen S, Jayaprakash B et al (2021) Diverse and active archaea communities occur in non-disinfected drinking water systems-less activity revealed in disinfected and hot water systems. Water Res 12:100101. https://doi.org/10.1016/j.wroa.2021.100101
Niu J, Kasuga I, Kurisu F et al (2013) Evaluation of autotrophic growth of ammonia-oxidizers associated with granular activated carbon used for drinking water purification by DNA-stable isotope probing. Water Res 47(19):7053–7065. https://doi.org/10.1016/j.watres.2013.07.056
Folkman S (2018) Water main break rates in the USA and Canada: a comprehensive study. Mech Aerospace Eng Fac Publ 174. https://digitalcommons.usu.edu/mae_facpub/174
Gomez-Smith CK, Lapara TM, Hozalski RM (2015) Sulfate reducing bacteria and mycobacteria dominate the biofilm communities in a chloraminated drinking water distribution system. Environ Sci Technol 49(14):8432–8440. https://doi.org/10.1021/acs.est.5b00555
Zhou C, Wu J, Dong L et al (2020) Removal of antibiotic resistant bacteria and antibiotic resistance genes in wastewater effluent by UV-activated persulfate. J Hazard Mater 388:122070. https://doi.org/10.1016/j.jhazmat.2020.122070
Li Q, Yu SL, Li L et al (2017) Microbial communities shaped by treatment processes in a drinking water treatment plant and their contribution and threat to drinking water safety. Front Microbiol 8:2465. https://doi.org/10.3389/fmicb.2017.02465
Zhang J, Kobert K, Flouri T et al (2014) PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30(5):614–620. https://doi.org/10.1093/bioinformatics/btt593
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998. https://doi.org/10.1038/nmeth.2604
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb 75(23):7537–7541. https://doi.org/10.1128/aem.01541-09
Shen D, Liu S, Zhu Q et al (2014) Distribution and diversity of nitrite-dependent anaerobic methane-oxidising bacteria in the sediments of the Qiantang River. Microb Ecol 67(2):341–349. https://doi.org/10.1007/s00248-013-0330-0
Langille MGI, Zaneveld J, Caporaso JG et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31(9):814–821. https://doi.org/10.1038/nbt.2676
Andrei A, Baricz A, Robeson II M et al (2017) Hypersaline sapropels act as hotsports for microbial dark matter. Sci. Rep. 7:6150. https://doi.org/10.1038/s41598-017-06232-w
Liu S, Gunawan C, Barraud N et al (2016) Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems. Environ. Sci. Technol. 50(17):8954–8976. https://doi.org/10.1021/acs.est.6b00835
Bal Krishana KC, Sathasivan A, Ginige MP (2013) Microbial community changes with decaying chloramine residuals in a lab-scale system. Water Research 47:4666–4679
Peng C, Korshin GV (2011) Speciation of trace inorganic contaminants in corrosion scales and deposits formed in drinking water distribution systems. Water Res 45(17):5553–5563. https://doi.org/10.1016/j.watres.2011.08.017
Li G, Ma X, Chen R et al (2019) Field studies of manganese deposition and release in drinking water distribution systems: insight into deposit control. Water Res 163:114897. https://doi.org/10.1016/j.watres.2019.114897
Zhang Y, Shi B, Zhao Y et al (2016) Deposition behavior of residual aluminum in drinking water distribution system: effect of aluminum speciation. J Environ Sci 42:142–151. https://doi.org/10.1016/j.jes.2015.05.010
Chen X, **ao L, Niu J et al (2023) Early succession of biofilm bacterial communities in newly built drinking water pipelines via multi-area analysis. Appl Microbiol Biot 107:3817–3828. https://doi.org/10.1007/s00253-023-12517-0
Huo L, Pan L, Chen R et al (2021) Effects of disinfectants and particles on the occurrence of different microorganisms in drinking water distribution systems. Environ Sci-Wat Res Technol 7:983–992. https://doi.org/10.1039/D0EW01119C
Nagymáté Z, Homonnay ZG, Márialigeti K (2016) Investigation of archaeal and bacterial community structure of five different small drinking water networks with special regard to the nitrifying microorganisms. Microbiol Res 188:80–89. https://doi.org/10.1016/j.micres.2016.04.015
Roy D, McEvoy J, Khan E (2020) Abundance and activity of ammonia oxidizing archaea and bacteria in bulk water and biofilm in water supply systems practicing chlorination and chloramination: full and laboratory scale investigations. Sci Total Environ 715:137043. https://doi.org/10.1016/j.scitotenv.2020.137043
Martens-Habbena W, STAHL DA (2011) Nitrogen metabolism and kinetics of ammonia-oxidizing archaea. Method Enzymol 496:465–487. https://doi.org/10.1016/B978-0-12-386489-5.00019-1
Han Z, An W, Yang M et al (2020) Assessing the impact of source water on tap water bacterial communities in 46 drinking water supply systems in China. Water Res 172:115469. https://doi.org/10.1016/j.watres.2020.115469
Li C, Wang S, Du X et al (2016) Immobilization of iron- and manganese-oxidizing bacteria with a biofilm-forming bacterium for the effective removal of iron and manganese from groundwater. Bioresource Technol 220:76–84. https://doi.org/10.1016/j.biortech.2016.08.020
Selleck EM, Tyne DV, Gilmore MS (2019) Pathogenicity of Enterococci. Microbiol Spectr 7(4). https://doi.org/10.1128/microbiolspec.GPP3-0053-2018
U.S. Environmental Protection Agency (2009) Method 1600: Enterococci in water by membrane filtration using membrane-Enterococcus indoxyl-β-D-Glucoside agar (mEI) No. EPA 821-R-09-016. Washington, DC: U.S. Environmental Protection Agency.
Learbuch KLG, Smidt H, van der Wielen PWJJ (2021) Influence of pipe materials on the microbial community in unchlorinated drinking water and biofilm. Water Res:116922. https://doi.org/10.1016/j.watres.2021.116922
Zhang X, Lin T, Jiang F et al (2022) Impact of pipe material and chlorination on the biofilm structure and microbial communities. Chemosphere 133218. https://doi.org/10.1016/j.chemosphere.2021.133218
Chen X, Wang Y, Li W et al (2020) Microbial contamination in distributed drinking water purifiers induced by water stagnation. Enviro Res 188:109715. https://doi.org/10.1016/j.envres.2020.109715
Li W, Tan Q, Zhou W et al (2020) Impact of substrate material and chlorine/chloramine on the composition and function of a young biofilm microbial community as revealed by high-throughput 16S rRNA sequencing. Chemosphere 242:125310. https://doi.org/10.1016/j.chemosphere.2019.125310
Chesney JA, Eaton JW, Mahoney JR (1996) Bacterial glutathione: a sacrificial defense against compounds. J Bacteriol 178(7):2131–2135. https://doi.org/10.1128/jb.178.7.2131-2135.1996
Hosseyni S, Jarrapour A (2018) Recent advances in beta-lactam synthesis. Org Biomol Chem 16(38):6840–6852
Allen HK, Donato J, Wang HH et al (2010) Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol 8(4):251–259. https://doi.org/10.1038/nrmicro2312
Funding
This work was supported by the National Natural Science Foundation of China (grant number 52000032); the Natural Science Foundation of Fujian Province (grant number 2023J01933); and Fuzhou Water Supply Co., Ltd. and Fuzhou Water Quality Monitoring Co., Ltd. (grant number GY-H-21258).
Author information
Authors and Affiliations
Contributions
J N conceived, performed research, and wrote the paper. D C analyzed data and wrote the paper. C S and L X performed research and analyzed data. Y W collected and analyzed data. W Z, X Z, Z C, and X D prepared material and performed research. X C conceived research and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
The nucleotide sequence data reported are available in the GenBank databases under the accession numbers SRR17325760 to SRR17325763; SRR18477630 to SRR18477634.
Supplementary Information
ESM 1
(DOCX 538 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niu, J., Chen, D., Shang, C. et al. Niche Differentiation of Biofilm Microorganisms in a Full-scale Municipal Drinking Water Distribution System in China and Their Implication for Biofilm Control. Microb Ecol 86, 2770–2780 (2023). https://doi.org/10.1007/s00248-023-02274-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02274-y