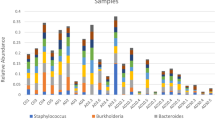
Abstract
A breakdown in host-bacteria relationships has been associated with the progression of a number of marine diseases and subsequent mortality events. For the Pacific oyster, Crassostrea gigas, summer mortality syndrome (SMS) is one of the biggest constraints to the growth of the sector and is set to expand into temperate systems as ocean temperatures rise. Currently, a lack of understanding of natural spatiotemporal dynamics of the host-bacteria relationship limits our ability to develop microbially based monitoring approaches. Here, we characterised the associated bacterial community of C. gigas, at two Irish oyster farms, unaffected by SMS, over the course of a year. We found C. gigas harboured spatiotemporally variable bacterial communities that were distinct from bacterioplankton in surrounding seawater. Whilst the majority of bacteria-oyster associations were transient and highly variable, we observed clear patterns of stability in the form of a small core consisting of six persistent amplicon sequence variants (ASVs). This core made up a disproportionately large contribution to sample abundance (34 ± 0.14%), despite representing only 0.034% of species richness across the study, and has been associated with healthy oysters in other systems. Overall, our study demonstrates the consistent features of oyster bacterial communities across spatial and temporal scales and provides an ecologically meaningful baseline to track environmental change.





Similar content being viewed by others
Data availability
Sequences are accessible through the EMBL database (accession no. PRJEB52444). ASV table and metadata are available at https://figshare.com/s/b36ed8e1872f496d437a.
References
Nyholm S, Graf J (2012) Knowing your friends: invertebrate innate immunity fosters beneficial bacterial symbioses. Nat Rev Microbiol 10:815–827
Egan S, Harder T, Burke C et al (2013) The seaweed holobiont: understanding seaweed-bacteria interactions. FEMS Microbiol Rev 37:462–476
Beinart RA (2019) The significance of microbial symbionts in ecosystem processes. mSystems 4:e00127-19
Wilkins LGE, Leray M, O’Dea A et al (2019) Host-associated microbiomes drive structure and function of marine ecosystems. PLoS Biol 17:e3000533
Vanwonterghem I, Webster NS (2020) Coral reef microorganisms in a changing climate. iScience 23:100972
Dittami SM, Arboleda E, Auguet JC et al (2021) A community perspective on the concept of marine holobionts: current status, challenges, and future directions. PeerJ 9:e10911
Hernandez-Agreda A, Leggat W, Bongaerts P et al (2018) Rethinking the coral microbiome: simplicity exists within a diverse microbial biosphere. mBio 9:e00812-18
Phelps CM, McMahon K, Bissett A et al (2021) The surface bacterial community of an Australian kelp shows cross-continental variation and relative stability within regions. FEMS Microbiology Ecology 97:fiab089
Shade A, Handelsman J (2012) Beyond the Venn diagram: the hunt for a core microbiome. Environ Microbiol 14:4–12
Pita L, Rix L, Slaby BM et al (2018) (2018) The sponge holobiont in a changing ocean: from microbes to ecosystems. Microbiome 6:1–18
Risely A (2020) Applying the core microbiome to understand host–microbe systems. J Anim Ecol 89:1549–1558
Björk JR, O’Hara RB, Ribes M, et al (2018) The dynamic core microbiome: structure, dynamics and stability. bioRxiv:137885.
Zaneveld JR, McMinds R, Thurber RV (2017) Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nature Microbiology 9(2):1–8
Glasl B, Bourne DG, Frade PR et al (2019) Microbial indicators of environmental perturbations in coral reef ecosystems. Microbiome 7:1–13
Egan S, Gardiner M (2016) Microbial dysbiosis: rethinking disease in marine ecosystems. Front Microbiol 7:991
Glasl B, Webster NS, Bourne DG (2017) Microbial indicators as a diagnostic tool for assessing water quality and climate stress in coral reef ecosystems. Mar Biol 164:1–18
Marzinelli EM, Campbell AH, Zozaya Valdes E et al (2015) Continental-scale variation in seaweed host-associated bacterial communities is a function of host condition, not geography. Environ Microbiol 17:4078–4088
Minich JJ, Morris MM, Brown M et al (2018) Elevated temperature drives kelp microbiome dysbiosis, while elevated carbon dioxide induces water microbiome disruption. PLoS ONE 13:e0192772
Scanes E, Parker LM, Seymour JR et al (2021) Climate change alters the haemolymph microbiome of oysters. Mar Pollut Bull 164:111991
Paillard C, le Roux F, Borrego JJ (2004) Bacterial disease in marine bivalves, a review of recent studies: trends and evolution. Aquat Living Resour 17:477–498
Soon TK, Zheng H (2019) Climate change and bivalve mass mortality in temperate regions. Rev Environ Contam Toxicol 251:109–129
Pernet F, Lupo C, Bacher C, Whittington RJ (2016) Infectious diseases in oyster aquaculture require a new integrated approach. Philosophical Transactions of the Royal Society B: Biological Sciences 371:20150213
Petton B, Destoumieux-Garzón D, Pernet F et al (2021) The Pacific oyster mortality syndrome, a polymicrobial and multifactorial disease: state of knowledge and future directions. Front Immunol 12:52
Malham SK, Cotter E, O’Keeffe S et al (2009) Summer mortality of the Pacific oyster, Crassostrea gigas, in the Irish Sea: the influence of temperature and nutrients on health and survival. Aquaculture 287:128–138
King WL, Jenkins C, Seymour JR, Labbate M (2019) Oyster disease in a changing environment: decrypting the link between pathogen, microbiome and environment. Mar Environ Res 143:124–140
Lasa A, di Cesare A, Tassistro G et al (2019) Dynamics of the Pacific oyster pathobiota during mortality episodes in Europe assessed by 16S rRNA gene profiling and a new target enrichment next-generation sequencing strategy. Environ Microbiol 21:4548–4562
Green TJ, Siboni N, King WL et al (2019) Simulated marine heat wave alters abundance and structure of Vibrio populations associated with the Pacific oyster resulting in a mass mortality event. Microb Ecol 77:736–747
King WL, Jenkins C, Go J et al (2019) Characterisation of the Pacific oyster microbiome during a summer mortality event. Microb Ecol 77:502–512
King WL, Siboni N, Kahlke T et al (2020) Regional and oyster micro-environmental scale heterogeneity in the Pacific oyster bacterial community. FEMS Microbiology Ecology 96:fiaa054
Ramsby BD, Hoogenboom MO, Whalan S, Webster NS (2018) Elevated seawater temperature disrupts the microbiome of an ecologically important bioeroding sponge. Mol Ecol 27:2124–2137
Qiu Z, Coleman MA, Provost E et al (2019) Future climate change is predicted to affect the microbiome and condition of habitat-forming kelp. Proc R Soc B 286:20181887
Go J, Deutscher AT, Spiers ZB et al (2017) Mass mortalities of unknown aetiology in Pacific oysters Crassostrea gigas in Port Stephens, New South Wales, Australia. Dis Aquat Org 125:227–242
Fleury E, Barbier P, Petton B et al (2020) Latitudinal drivers of oyster mortality: deciphering host, pathogen and environmental risk factors. Sci Rep 10:1–12
Rowley AF, Cross ME, Culloty SC et al (2014) The potential impact of climate change on the infectious diseases of commercially important shellfish populations in the Irish Sea—a review. ICES J Mar Sci 71:741–759
Kozich JJ, Westcott SL, Baxter NT et al (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112
Callahan BJ, Sankaran K, Fukuyama JA et al (2016) Bioconductor workflow for microbiome data analysis: from raw reads to community analyses. F1000Res 5:1492
Quast C, Pruesse E, Yilmaz P et al (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8.
CHAO A (1984) Non-parametric estimation of the number of classes in a population. Scandinavian journal of statistics. 265–270
Oksanen J, Blanchet FG, Friendly M et al (2019) Package “vegan”. Community Ecology Package. Community ecology package 2
Nguyen VK, King WL, Siboni N et al (2020) The Sydney rock oyster microbiota is influenced by location, season and genetics. Aquaculture 527:735472
Unzueta-Martínez A (2022) Determining the composition of resident and transient members of the oyster microbiome. Front Microbiol 12:4350
Lokmer A, Mathias Wegner K (2014) Hemolymph microbiome of Pacific oysters in response to temperature, temperature stress and infection. The ISME Journal 3(9):670–682
Stevick RJ, Post AF, Gómez-Chiarri M (2021) Functional plasticity in oyster gut microbiomes along a eutrophication gradient in an urbanized estuary. Animal Microbiome 1(3):1–17
Pierce ML, Ward JE (2019) Gut microbiomes of the eastern oyster (Crassostrea virginica) and the blue mussel (Mytilus edulis): temporal variation and the influence of marine aggregate-associated microbial communities. mSphere 4:e00730–e00719
Chase JM, Myers JA (2011) Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B Biol Sci 366:2351–2363
Lokmer A, Goedknegt MA, Thieltges DW et al (2016) Spatial and temporal dynamics of Pacific oyster haemolymph microbiota across multiple scales. Front Microbiol 7:1367
Bunse C, Pinhassi J (2017) Marine bacterioplankton seasonal succession dynamics. Trends Microbiol 25:494–505
Ladau J, Sharpton TJ, Finucane MM et al (2013) Global marine bacterial diversity peaks at high latitudes in winter. The ISME Journal 9(7):1669–1677
García FC, Alonso-Sáez L, Morán XAG, López-Urrutia Á (2015) Seasonality in molecular and cytometric diversity of marine bacterioplankton: the re-shuffling of bacterial taxa by vertical mixing. Environ Microbiol 17:4133–4142
Gilbert JA, Steele JA, Caporaso JG et al (2011) Defining seasonal marine microbial community dynamics. The ISME Journal 2(6):298–308
Pierce ML, Ward JE, Holohan BA et al (2016) The influence of site and season on the gut and pallial fluid microbial communities of the eastern oyster, Crassostrea virginica (Bivalvia, Ostreidae): community-level physiological profiling and genetic structure. Hydrobiologia 765:97–113
Avila-Poveda OH, Torres-Ariño A, Girón-Cruz DA, Cuevas-Aguirre A (2014) Evidence for accumulation of Synechococcus elongatus (Cyanobacteria: Cyanophyceae) in the tissues of the oyster Crassostrea gigas (Mollusca: Bivalvia). Tissue Cell 46:379–387
King GM, Judd C, Kuske CR, Smith C (2012) Analysis of stomach and gut microbiomes of the eastern oyster (Crassostrea virginica) from coastal Louisiana, USA. PLoS ONE 7:e51475
Pimentel ZT, Dufault-Thompson K, Russo KT et al (2021) Microbiome analysis reveals diversity and function of Mollicutes associated with the eastern oyster, Crassostrea virginica. mSphere 6
Green TJ, Barnes AC (2010) Bacterial diversity of the digestive gland of Sydney rock oysters, Saccostrea glomerata infected with the paramyxean parasite, Marteilia sydneyi. J Appl Microbiol 109:613–622
Acknowledgements
We thank oyster farmers at Bannow Bay and Cromane for their continued support and facilitation of this research.
Funding
This research was funded by the European Regional Development fund through the Interreg Ireland Wales Cooperation Programme project BLUEFISH and the EU’s West Wales and the Valleys project SHELLFISH CENTRE.
Author information
Authors and Affiliations
Contributions
NGK and SKM conceived the designed the study. JT conducted all laboratory work. RB and AA conducted all fieldwork. NGK lead the manuscript preparation, and all authors contributed equally to subsequent edits. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Declaration
No approval of research ethics committees was required to accomplish the goals of this study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
King, N.G., Smale, D.A., Thorpe, J.M. et al. Core Community Persistence Despite Dynamic Spatiotemporal Responses in the Associated Bacterial Communities of Farmed Pacific Oysters. Microb Ecol 86, 154–162 (2023). https://doi.org/10.1007/s00248-022-02083-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-022-02083-9