Abstract
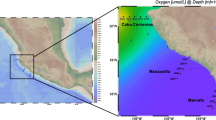
The environmental variations and their interactions with the biosphere are vital in the Arctic Ocean during the summer sea-ice melting period in the current scenario of climate change. Hence, we analysed the vertical distribution of bacterial and archaeal communities in the western Arctic Ocean from sea surface melt-ponds to deep water up to a 3040 m depth. The distribution of microbial communities showed a clear stratification with significant differences among different water depths, and the water masses in the Arctic Ocean – surface mixed layer, Atlantic water mass and deep Arctic water – appeared as a major factor explaining their distribution in the water column. A total of 34 bacterial phyla were detected in the seawater and 10 bacterial phyla in melt-ponds. Proteobacteria was the dominant phyla in the seawater irrespective of depth, whereas Bacteroidota was the dominant phyla in the melt-ponds. A fast expectation-maximization microbial source tracking analysis revealed that only limited dispersion of the bacterial community was possible across the stratified water column. The surface water mass contributed 21% of the microbial community to the deep chlorophyll maximum (DCM), while the DCM waters contributed only 3% of the microbial communities to the deeper water masses. Atlantic water mass contributed 37% to the microbial community of the deep Arctic water. Oligotrophic heterotrophic bacteria were dominant in the melt-ponds and surface waters, whereas chemoautotrophic and mixotrophic bacterial and archaeal communities were abundant in deeper waters. Chlorophyll and ammonium were the major environmental factors that determined the surface microbial communities, whereas inorganic nutrient concentrations controlled the deep-water communities.







Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available in the NCBI repository, Bioproject number PRJNA770954). Submission information can be found at: http://www.ncbi.nlm.nih.gov/sra
Code Availability
Codes used in the current study are available from the corresponding author on reasonable request.
References
Laxon S, Peacock N, Smith D (2003) High interannual variability of sea ice thickness in the Arctic region. Nature 425:947–950
Frey K, Comiso JC, Cooper LW, Eisner LB, Gradinger RR, Grebmeier JM, JE Tremblay (2017) Arctic Ocean Primary Productivity. In: Arctic Report Card 2017, NOAA, http://www.arctic.noaa.gov/Report-Card/Report-Card-2017/ArtMID/7798/ArticleID/701/Arctic-Ocean-Primary-Productivity
Barber DG, Hop H, Mundy CJ, Else B, Dmitrenko IA, Tremblay J-E, Ehn JK, Assmy P, Daase M, Candlish LM (2015) Selected physical, biological and biogeochemical implications of a rapidly changing Arctic Marginal Ice Zone. Prog Oceanogr 139:122–150
Pemberton P, Nilsson J, Hieronymus M, Meier HM (2015) Arctic Ocean water mass transformation in S–T coordinates. J Phys Oceanography 45:1025–1050
Rudels B (2009) Arctic ocean circulation. Academic, New York
Boé J, Hall A, Qu X (2009) September sea-ice cover in the Arctic Ocean projected to vanish by 2100. Nature Geoscience 2:341–343
Wang M, Overland JE (2009) A sea ice free summer Arctic within 30 years? Geophys Res Lett 36. https://doi.org/10.1029/2009GL037820
Strong C, Foster D, Cherkaev E, Eisenman I, Golden KM (2017) On the definition of marginal ice zone width. J Atmospheric Oceanic Technol 34:1565–1584
Rolph RJ, Feltham DL, Schröder D (2020) Changes of the Arctic marginal ice zone during the satellite era. Cryosphere 14:1971–1984
Collins III CO, Rogers WE, Marchenko A, Babanin AV (2015) In situ measurements of an energetic wave event in the Arctic marginal ice zone. Geophys Res Lett 42:1863–1870
Squire VA (2007) Of ocean waves and sea-ice revisited. Cold Regions Sci Technol 49:110–133
Mundy C, Gosselin M, Ehn JK, Belzile C, Poulin M, Alou E, Roy S, Hop H, Lessard S, Papakyriakou TN (2011) Characteristics of two distinct high-light acclimated algal communities during advanced stages of sea ice melt. Polar Biol 34:1869–1886
DeLong EF, Preston CM, Mincer T, Rich V, Hallam SJ, Frigaard N-U, Martinez A, Sullivan MB, Edwards R, Brito BR (2006) Community genomics among stratified microbial assemblages in the ocean’s interior. Science 311:496–503
Brown MV, Philip GK, Bunge JA, Smith MC, Bissett A, Lauro FM, Fuhrman JA, Donachie SP (2009) Microbial community structure in the North Pacific ocean. ISME J 3:1374–1386
Agogué H, Lamy D, Neal PR, Sogin ML, Herndl GJ (2011) Water mass-specificity of bacterial communities in the North Atlantic revealed by massively parallel sequencing. Mol Ecol 20:258–274
Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M (2014) Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PloS One 9:e105592
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596
Chong J, Liu P, Zhou G, **a J (2020) Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nature Protocols 15:799–821
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RLV, Knight R (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnol 31:814–821
Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MG (2020) PICRUSt2 for prediction of metagenome functions. Nature Biotechnol 38:685–688
Team R (2015) RStudio: integrated development for R. RStudio, Inc, Boston, MA URL http://www.rstudio.com 42: 14.
Shenhav L, Thompson M, Joseph TA, Briscoe L, Furman O, Bogumil D, Mizrahi I, Pe’er I, Halperin E (2019) FEAST: fast expectation-maximization for microbial source tracking. Nature Methods 16:627
Ter Braak CJ, Smilauer P (2002) CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5). www.canoco.com .
Harrell Jr FE, Harrell Jr MFE (2019) Package ‘Hmisc’. CRAN2018 2019: 235-236
Giovannoni SJ, Tripp HJ, Givan S, Podar M, Vergin KL, Baptista D, Bibbs L, Eads J, Richardson TH, Noordewier M (2005) Genome streamlining in a cosmopolitan oceanic bacterium. Science 309:1242–1245
**g H, **a X, Suzuki K, Liu H (2013) Vertical profiles of bacteria in the tropical and subarctic oceans revealed by pyrosequencing. PloS One 8:e79423
Dobal-Amador V, Nieto-Cid M, Guerrero-Feijoo E, Hernando-Morales V, Teira E, Varela MM (2016) Vertical stratification of bacterial communities driven by multiple environmental factors in the waters (0–5000 m) off the Galician coast (NW Iberian margin). Deep Sea Res Part I: Oceanographic Res Papers 114:1–11
Walsh EA, Kirkpatrick JB, Rutherford SD, Smith DC, Sogin M, D'Hondt S (2016) Bacterial diversity and community composition from seasurface to subseafloor. ISME J 10:979–989
Nishino S, Kikuchi T, Yamamoto-Kawai M, Kawaguchi Y, Hirawake T, Itoh M (2011) Enhancement/reduction of biological pump depends on ocean circulation in the sea-ice reduction regions of the Arctic Ocean. J Oceanography 67:305–314
Lalande C, Nöthig EM, Fortier L (2019) Algal export in the Arctic Ocean in times of global warming. Geophys Res Lett 46:5959–5967
Lalande C, Forest A, Barber DG, Gratton Y, Fortier L (2009) Variability in the annual cycle of vertical particulate organic carbon export on Arctic shelves: Contrasting the Laptev Sea, Northern Baffin Bay and the Beaufort Sea. Continental Shelf Res 29:2157–2165
Cai P, Rutgers Van Der Loeff M, Stimac I, Nöthig EM, Lepore K, Moran S (2010) Low export flux of particulate organic carbon in the central Arctic Ocean as revealed by 234Th: 238U disequilibrium. J Geophys Res: Oceans 115. https://doi.org/10.1029/2009JC005595
Cai W-J, Chen L, Chen B, Gao Z, Lee SH, Chen J, Pierrot D, Sullivan K, Wang Y, Hu X (2010) Decrease in the CO2 uptake capacity in an ice-free Arctic Ocean basin. Science 329:556–559
Han D, Ha HK, Hwang CY, Lee BY, Hur H-G, Lee YK (2015) Bacterial communities along stratified water columns at the Chukchi Borderland in the western Arctic Ocean. Deep Sea Res Part II: Topical Stud Oceanogr 120:52–60
Fu Y, MacLeod DM, Rivkin RB, Chen F, Buchan A, Lang AS (2010) High diversity of Rhodobacterales in the subarctic North Atlantic Ocean and gene transfer agent protein expression in isolated strains. Aquatic Microbial Ecol 59:283–293
Raes EJ, Bodrossy L, Van De Kamp J, Bissett A, Ostrowski M, Brown MV, Sow SL, Sloyan B, Waite AM (2018) Oceanographic boundaries constrain microbial diversity gradients in the South Pacific Ocean. Proc Natl Acad Sci 115:E8266–E8275
Milici M, Tomasch J, Wos-Oxley ML, Wang H, Jáuregui R, Camarinha-Silva A, Deng Z-L, Plumeier I, Giebel H-A, Wurst M (2016) Low diversity of planktonic bacteria in the tropical ocean. Scientific Reports 6:1–9
Montes T, Guerrero-Feijóo E, Moreira-Coello V, Bode A, Ruiz-Villarreal M, Mouriño-Carballido B, Varela MM (2020) Vertical zonation of bacterial assemblages attributed to physical stratification during the summer relaxation of the coastal upwelling off Galicia (NW Spain). Estuarine, Coastal Shelf Sci 245:106791
Schattenhofer M, Fuchs BM, Amann R, Zubkov MV, Tarran GA, Pernthaler J (2009) Latitudinal distribution of prokaryotic picoplankton populations in the Atlantic Ocean. Environ Microbiol 11:2078–2093
Cottrell MT, Kirchman DL (2000) Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low-and high-molecular-weight dissolved organic matter. Appl Environ Microbiol 66:1692–1697
Brown MV, Bowman JP (2001) A molecular phylogenetic survey of sea-ice microbial communities (SIMCO). FEMS Microbiol Ecol 35:267–275
Maccario L, Carpenter SD, Deming JW, Vogel TM, Larose C (2019) Sources and selection of snow-specific microbial communities in a Greenlandic sea ice snow cover. Scientific Reports 9:1–14
Sørensen HL, Thamdrup B, Jeppesen E, Rysgaard S, Glud RN (2017) Nutrient availability limits biological production in Arctic sea ice melt ponds. Polar Biol 40:1593–1606
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49
Avcı B, Krüger K, Fuchs BM, Teeling H, Amann RI (2020) Polysaccharide niche partitioning of distinct Polaribacter clades during North Sea spring algal blooms. ISME J 14:1369–1383
Bryant JA, Stewart FJ, Eppley JM, DeLong EF (2012) Microbial community phylogenetic and trait diversity declines with depth in a marine oxygen minimum zone. Ecology 93:1659–1673
Lindh MV, Maillot BM, Shulse CN, Gooday AJ, Amon DJ, Smith CR, Church MJ (2017) From the surface to the deep-sea: bacterial distributions across polymetallic nodule fields in the clarion-clipperton zone of the Pacific Ocean. Front Microbiol 8:1696
Balmonte JP, Teske A, Arnosti C (2018) Structure and function of high Arctic pelagic, particle-associated and benthic bacterial communities. Environ Microbiol 20:2941–2954
Qian P-Y, Wang Y, Lee OO, Lau SC, Yang J, Lafi FF, Al-Suwailem A, Wong TY (2011) Vertical stratification of microbial communities in the Red Sea revealed by 16S rDNA pyrosequencing. ISME J 5:507–518
Hansell DA (2013) Recalcitrant dissolved organic carbon fractions. Ann Rev Mar Sci 5(1):421–445
Herndl GJ, Reinthaler T (2013) Microbial control of the dark end of the biological pump. Nature Geosci 6:718–724
Swan BK, Martinez-Garcia M, Preston CM, Sczyrba A, Woyke T, Lamy D, Reinthaler T, Poulton NJ, Masland EDP, Gomez ML (2011) Potential for chemolithoautotrophy among ubiquitous bacteria lineages in the dark ocean. Science 333:1296–1300
Tang K, Yang Y, Lin D, Li S, Zhou W, Han Y, Liu K, Jiao N (2016) Genomic, physiologic, and proteomic insights into metabolic versatility in Roseobacter clade bacteria isolated from deep-sea water. Scientific Reports 6:35528
Landry Z, Swan BK, Herndl GJ, Stepanauskas R, Giovannoni SJ (2017) SAR202 genomes from the dark ocean predict pathways for the oxidation of recalcitrant dissolved organic matter. MBio 8:e00413–17
Rinke C, Rubino F, Messer LF, Youssef N, Parks DH, Chuvochina M, Brown M, Jeffries T, Tyson GW, Seymour JR (2019) A phylogenomic and ecological analysis of the globally abundant Marine Group II archaea (Ca. Poseidoniales ord. nov.). ISME J 13:663–675
Zhang CL, **e W, Martin-Cuadrado A-B, Rodriguez-Valera F (2015) Marine Group II Archaea, potentially important players in the global ocean carbon cycle. Front Microbiol 6:1108
Middelburg JJ (2011) Chemoautotrophy in the ocean. Geophys Res Lett 38(L24604):1–4
Acknowledgements
The authors wish to express their sincere gratitude to the Director, NCPOR, Ministry of Earth Sciences, for providing the necessary facilities for carrying out this research. This research was a part of the project titled ‘Korea-Arctic Ocean Warming and Response of Ecosystem (K-AWARE, KOPRI, 1525011760)’, funded by the Ministry of Oceans and Fisheries, Korea. The authors also thank Asian Forum for Polar Sciences (AFOPS) for facilitating the Arctic expedition 2019. The authors thank the captain and crew of the IBRV ARAON who were most helpful in all shipboard operations. This is NCPOR contribution number J-84/ 2021-22.
Funding
This work was supported by the Ministry of Earth Sciences, Govt. of India and Ministry of Oceans and Fisheries, Republic of Korea. Grant numbers [K-AWARE, KOPRI, 1525011760]. Authors Puthiya Veettil Vipindas, Siddarthan Venkatachalam, Thajudeen Jabir and Kottekkatu Padinchati Krishnan have received research support from NCPOR, India. Authors Eun ** Yang, Kyoung-Ho Cho, **young Jung and Youngju Lee have received research support from KOPRI, Korea.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Puthiya Veettil Vipindas, Siddarthan Venkatachalam, Thajudeen Jabir, Eun ** Yang, Kyoung-Ho Cho, **young Jung and Youngju Lee. The first draft of the manuscript was written by Puthiya Veettil Vipindas and edited by Kottekkatu Padinchati Krishnan. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
This manuscript does not contain any studies with human participants
Consent to Publish
This manuscript does not contain any studies with human participants
Rights and permissions
About this article
Cite this article
Vipindas, P.V., Venkatachalam, S., Jabir, T. et al. Water Mass Controlled Vertical Stratification of Bacterial and Archaeal Communities in the Western Arctic Ocean During Summer Sea-Ice Melting. Microb Ecol 85, 1150–1163 (2023). https://doi.org/10.1007/s00248-022-01992-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-022-01992-z