Abstract
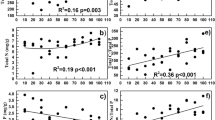
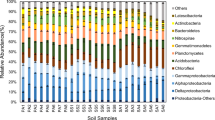
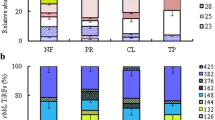
Photosynthetic microorganisms are widely distributed in the soil and play an important role in plant-free soil crusts. However, the distribution and environmental drivers of phototrophic microbial communities in physical soil crusts, where the abundance of cyanobacteria is low, are scarcely understood. Here, we performed high-throughput sequencing of pufM and 18S rRNA genes in soil crusts at different elevations on the Tibetan Plateau and used the data combined with environmental variables to analyze the diversity and structure of phototrophic microbial communities. We found that the dominant taxa of aerobic anoxygenic phototrophic bacteria (AAPB) and eukaryotic phototrophic microorganisms (EPM) were shown to shift with elevation. The phototrophic microbial diversity showed a single-peak pattern, with the lowest diversity of AAPB and highest diversity of EPM at middle elevations. Moreover, the elevation and soil property determined the phototrophic microbial community. Soil salts, especially Cl−, were the most important for AAPB. Likewise, soil nutrients, especially carbon, were the most important for EPM. The relationship between high-abundance taxa and environmental variables showed that Rhizobiales was significantly negatively correlated with salt ions and positively correlated with chlorophyll. Rhodobacterales showed the strongest and significant positive associations with Cl−. Chlorophyceae and Bacillariophyceae were positively correlated with CO32−. These results indicated that salinity and soil nutrients affected the diversity and structure of microbial communities. This study contributes to our understanding of the diversity, composition, and structure of photosynthetic microorganisms in physical soil crusts and helps in develo** new approaches for controlling desertification and salinization and improving the desert ecological environment.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon request. All sequence data generated by this study have been submitted to the NCBI Short Read Archive under BioProject PRJNA669450 with accession number SUB8323950.
References
West NE (1990) Structure and Function of Microphytic Soil Crusts in Wildland Ecosystems of Arid to Semi-arid Regions. Adv Ecol Res 20:179–223. https://doi.org/10.1016/S0065-2504(08)60055-0
Belnap J, Weber B, Büdel B (2016) In: Weber B, Büdel B, Belnap J (eds) Biological soil crusts: an organizing principle in drylands. Springer, Cham, pp 3–13. https://doi.org/10.1007/978-3-319-30214-0_1
Ferrenberg S, Reed SC, Belnap J (2015) Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc Natl Acad Sci U S A 112(39):12116–12121. https://doi.org/10.1073/pnas.1509150112
Leung PM, Bay SK, Meier DV, Chiri E, Cowan DA, Gillor O, Woebken D, Greening C (2020). mSystems 5(2):e00495–e00419. https://doi.org/10.1128/mSystems.00495-19
Li H, Huo D, Wang W, Chen Y, Cheng X, Yu G, Li R (2020) Multifunctionality of biocrusts is positively predicted by network topologies consistent with interspecies facilitation. Mol Ecol 29(8):1560–1573. https://doi.org/10.1111/mec.15424
Bryant DA, Frigaard NU (2006) Prokaryotic photosynthesis and phototrophy illuminated. Trends Microbiol 14(11):488–496. https://doi.org/10.1016/j.tim.2006.09.001
Csotonyi JT, Swiderski J, Stackebrandt E, Yurkov VV (2008) Novel halophilic aerobic anoxygenic phototrophs from a Canadian hypersaline spring system. Extremophiles 12(4):529–539. https://doi.org/10.1007/s00792-008-0156-8
Ooms MD, Dinh CT, Sargent EH, Sinton D (2016) Photon management for augmented photosynthesis. Nat Commun 7:12699. https://doi.org/10.1038/ncomms12699
VanInsberghe D, Maas KR, Cardenas E, Strachan CR, Hallam SJ, Mohn WW (2015) Non-symbiotic Bradyrhizobium ecotypes dominate North American forest soils. ISME J 9(11):2435–2441. https://doi.org/10.1038/ismej.2015.54
Tahon G, Tytgat B, Stragier P, Willems A (2016) Analysis of cbbL, nifH, and pufLM in Soils from the Sør Rondane Mountains, Antarctica, Reveals a Large Diversity of Autotrophic and Phototrophic Bacteria. Microb Ecol 71(1):131–149. https://doi.org/10.1007/s00248-015-0704-6
Yurkov V, Csotonyi J (2009) In: The purple phototrophic bacteria. Springer Netherlands, pp 31-55. doi:https://doi.org/10.1007/978-1-4020-8815-5_3
Tang K, Jia LJ, Yuan B, Yang SS, Li H, Meng J, Zeng Y, Feng F (2018) Aerobic Anoxygenic Phototrophic Bacteria Promote the Development of Biological Soil Crusts. Front Microbiol 9:2715. https://doi.org/10.3389/fmicb.2018.02715
Li K, Liu R, Zhang H, Yun J (2013) The Diversity and Abundance of Bacteria and Oxygenic Phototrophs in Saline Biological Desert Crusts in **njiang, Northwest China. Microb Ecol 66(1):40–48. https://doi.org/10.1007/s00248-012-0164-1
Koblizek M (2015) Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol Rev 39(6):854–870. https://doi.org/10.1093/femsre/fuv032
Garcia-Chaves MC, Cottrell MT, Kirchman DL, Ruiz-Gonzalez C, Del Giorgio PA (2016) Single-cell activity of freshwater aerobic anoxygenic phototrophic bacteria and their contribution to biomass production. ISME J 10(7):1579–1588. https://doi.org/10.1038/ismej.2015.242
Jiang HC, Dong HL, Yu BS, Lv G, Deng SC, Wu YJ, Dai MG, Jiao NZ (2009) Abundance and diversity of aerobic anoxygenic phototrophic bacteria in saline lakes on the Tibetan plateau. FEMS Microbiol Ecol 67(2):268–278. https://doi.org/10.1111/j.1574-6941.2008.00616.x
Hamilton TL, Bennett AC, Murugapiran SK, Havig JR (2019). mSystems 4(6):e00498–e00419. https://doi.org/10.1128/mSystems.00498-19
Ferrera I, Sarmento H, Priscu JC, Chiuchiolo A, Gonzalez JM, Grossart HP (2017) Diversity and Distribution of Freshwater Aerobic Anoxygenic Phototrophic Bacteria across a Wide Latitudinal Gradient. Front Microbiol 8:175. https://doi.org/10.3389/fmicb.2017.00175
Zhang K, Shi Y, Cui X, Yue P, Li K, Liu X, Tripathi BM, Chu H (2019). mSystems 4(1):e00225–e00218. https://doi.org/10.1128/mSystems.00225-18
Csotonyi JT, Swiderski J, Stackebrandt E, Yurkov V (2010) A new environment for aerobic anoxygenic phototrophic bacteria: biological soil crusts. Environ Microbiol Rep 2(5):651–656. https://doi.org/10.1111/j.1758-2229.2010.00151.x
Tahon G, Willems A (2017) Isolation and characterization of aerobic anoxygenic phototrophs from exposed soils from the Sør Rondane Mountains, East Antarctica. Syst Appl Microbiol 40(6):357–369. https://doi.org/10.1016/j.syapm.2017.05.007
da Rocha UN, Cadillo-Quiroz H, Karaoz U, Rajeev L, Klitgord N, Dunn S, Truong V, Buenrostro M, Bowen BP, Garcia-Pichel F, Mukhopadhyay A, Northen TR, Brodie EL (2015) Isolation of a significant fraction of non-phototroph diversity from a desert Biological Soil Crust. Front Microbiol 6:277. https://doi.org/10.3389/fmicb.2015.00277
Körner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22(11):569–574. https://doi.org/10.1016/j.tree.2007.09.006
Van der Rest M, Gingras G (1974) The Pigment Complement of the Photosynthetic Reaction Center Isolated from Rhodospirillum rubrum. J Biol Chem 249(20):6446–6453. https://doi.org/10.1016/S0021-9258(19)42177-7
**ong JB, Sun HB, Peng F, Zhang HY, Xue X, Gibbons SM, Gilbert JA, Chu HY (2014) Characterizing changes in soil bacterial community structure in response to short-term warming. FEMS Microbiol Ecol 89(2):281–292. https://doi.org/10.1111/1574-6941.12289
Zhou XB, Tao Y, Yin BF, Tucker C, Zhang YM (2020) Nitrogen pools in soil covered by biological soil crusts of different successional stages in a temperate desert in Central Asia. Geoderma 366:114166. https://doi.org/10.1016/j.geoderma.2019.114166
Zhou W, Jiang X, Ouyang J, Lu B, Liu W, Liu G (2020) Environmental Factors, More Than Spatial Distance, Explain Community Structure of Soil Ammonia-Oxidizers in Wetlands on the Qinghai–Tibetan Plateau. Microorganisms 8(6):933. https://doi.org/10.3390/microorganisms8060933
Mingorance MD, Barahona E, Fernandez-Galvez J (2007) Guidelines for improving organic carbon recovery by the wet oxidation method. Chemosphere 68(3):409–413. https://doi.org/10.1016/j.chemosphere.2007.01.021
Abed RMM, Al Kharusi S, Schramm A, Robinson MD (2010) Bacterial diversity, pigments and nitrogen fixation of biological desert crusts from the Sultanate of Oman. FEMS Microbiol Ecol 72(3):418–428. https://doi.org/10.1111/j.1574-6941.2010.00854.x
Yutin N, Suzuki MT, Beja O (2005) Novel Primers Reveal Wider Diversity among Marine Aerobic Anoxygenic Phototrophs. Appl Environ Microbiol 71(12):8958–8962. https://doi.org/10.1128/AEM.71.12.8958-8962.2005
Waidner LA, Kirchman DL (2008) Diversity and Distribution of Ecotypes of the Aerobic Anoxygenic Phototrophy Gene pufM in the Delaware Estuary. Appl Environ Microbiol 74(13):4012–4021. https://doi.org/10.1128/AEM.02324-07
Keck F, Millet L, Debroas D, Etienne D, Galop D, Rius D, Domaizon I (2020) Assessing the response of micro-eukaryotic diversity to the Great Acceleration using lake sedimentary DNA. Nat Commun 11(1):3831. https://doi.org/10.1038/s41467-020-17682-8
Luo ZM, Liu JX, Zhao PY, Jia T, Li C, Chai BF (2019) Biogeographic Patterns and Assembly Mechanisms of Bacterial Communities Differ Between Habitat Generalists and Specialists Across Elevational Gradients. Front Microbiol 10:169. https://doi.org/10.3389/fmicb.2019.00169
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin P, O’Hara RB, Simpson G, Solymos P, Stevens MHH, Wagner H (2016) Vegan: Community Ecology Package, R package version 2.3-5. https://cran.r-project.org/src/contrib/Archive/vegan/
Shannon CE (1948) A Mathematical Theory of Communication. Bell Syst Tech J 27(3):379–423. https://doi.org/10.1002/j.1538-7305.1948.tb01338.x
Chao A, Shen TJ (2003). Environ Ecol Stat 10(4):429–443. https://doi.org/10.1023/A:1026096204727
Simpson EH (1949) Measurement of Diversity. Nature 163(4148):688–688. https://doi.org/10.1038/163688a0
Yang YF, Gao Y, Wang SP, Xu DP, Yu H, Wu LW, Lin QY, Hu YG, Li XZ, He ZL, Deng Y, Zhou JZ (2014) The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J 8(2):430–440. https://doi.org/10.1038/ismej.2013.146
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) VARIATION PARTITIONING OF SPECIES DATA MATRICES: ESTIMATION AND COMPARISON OF FRACTIONS. Ecology 87(10):2614–2625. https://doi.org/10.1890/0012-9658(2006)87[2614:vposdm]2.0.co;2
McArdle BH, Anderson MJ (2001) FITTING MULTIVARIATE MODELS TO COMMUNITY DATA: A COMMENT ON DISTANCE-BASED REDUNDANCY ANALYSIS. Ecology 82(1):290–297. https://doi.org/10.1890/0012-9658(2001)082[0290:Fmmtcd]2.0.Co;2
De'ath G (2001) Multivariate Regression Trees: A New Technique for Modeling Species-Environment Relationships. Ecology 83:1105–1117. https://doi.org/10.2307/3071917
Praeg N, Seeber J, Leitinger G, Tasser E, Newesely C, Tappeiner U, Illmer P (2020) The role of land management and elevation in sha** soil microbial communities: Insights from the Central European Alps. Soil Biol Biochem 150:107951. https://doi.org/10.1016/j.soilbio.2020.107951
Wickham H (2009) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. https://doi.org/10.1007/978-0-387-98141-3
Shao JL, Lai B, Jiang W, Wang JT, Hong YH, Chen FB, Tan SQ, Guo LX (2019) Diversity and Co-Occurrence Patterns of Soil Bacterial and Fungal Communities of Chinese Cordyceps Habitats at Shergyla Mountain, Tibet: Implications for the Occurrence. Microorganisms 7(9):284. https://doi.org/10.3390/microorganisms7090284
Rui J, Li J, Wang S, An J, Liu W-T, Lin Q, Yang Y, He Z, Li X (2015). Appl Environ Microbiol 81(17):6070. https://doi.org/10.1128/AEM.00557-15
Velasco Ayuso S, Onatibia GR, Maestre FT, Yahdjian L (2020) Grazing pressure interacts with aridity to determine the development and diversity of biological soil crusts in Patagonian rangelands. Land Degrad Dev 31(4):488–499. https://doi.org/10.1002/ldr.3465
Hou J, Wu L, Liu W, Ge Y, Mu T, Zhou T, Li Z, Zhou J, Sun X, Luo Y, Christie P (2020) Biogeography and diversity patterns of abundant and rare bacterial communities in rice paddy soils across China. Sci Total Environ 730:139116. https://doi.org/10.1016/j.scitotenv.2020.139116
Angel R, Conrad R (2013) Elucidating the microbial resuscitation cascade in biological soil crusts following a simulated rain event. Environ Microbiol 15(10):2799–2815. https://doi.org/10.1111/1462-2920.12140
Yeoh YK, Dennis PG, Paungfoo-Lonhienne C, Weber L, Brackin R, Ragan MA, Schmidt S, Hugenholtz P (2017) Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence. Nat Commun 8(1):215. https://doi.org/10.1038/s41467-017-00262-8
Biebl H, Allgaier M, Tindall BJ, Koblizek M, Lunsdorf H, Pukall R, Wagner-Dobler I (2005) Dinoroseobacter shibae gen. nov., sp. nov., a new aerobic phototrophic bacterium isolated from dinoflagellates. Int J Syst Evol Microbiol 55:1089–1096. https://doi.org/10.1099/ijs.0.63511-0
Bill N, Tomasch J, Riemer A, Muller K, Kleist S, Schmidt-Hohagen K, Wagner-Dobler I, Schomburg D (2017) Fixation of CO2using the ethylmalonyl-CoA pathway in the photoheterotrophic marine bacteriumDinoroseobacter shibae. Environ Microbiol 19(7):2645–2660. https://doi.org/10.1111/1462-2920.13746
Liu W, Jiang HC, Yang J, Wu G (2018) Salinity and DOC Influence the Distribution of Free-living and Particle-attached Aerobic Anoxygenic Phototrophic Bacteria in the Qinghai–Tibetan Lakes. Geomicrobiol J 35(3):247–254. https://doi.org/10.1080/01490451.2017.1364805
Evans RD, Lange OL (2001) In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Ecological Studies. Springer, Berlin, pp 263–279. https://doi.org/10.1007/978-3-642-56475-8_20
Flechtner VR, Johansen JR, Clark WH (1998). Great Basin Nat 58(4):295–311
Pitschmann H (1963) Vorarbeiten zu einer monographie der gattung Heterococcus. Nova Hedwgia 5:487–531
Hollister EB, Engledow AS, Hammett AJM, Provin TL, Wilkinson HH, Gentry TJ (2010) Shifts in microbial community structure along an ecological gradient of hypersaline soils and sediments. ISME J 4(6):829–838. https://doi.org/10.1038/ismej.2010.3
Ben-Amotz A, Avron M (1973) The Role of Glycerol in the Osmotic Regulation of the Halophilic AlgaDunaliella parva. Plant Physiol 51(5):875–878. https://doi.org/10.1104/pp.51.5.875
Abed RMM, Tamm A, Hassenruck C, Al-Rawahi AN, Rodriguez-Caballero E, Fiedler S, Maier S, Weber B (2019) Habitat-dependent composition of bacterial and fungal communities in biological soil crusts from Oman. Sci Rep 9(1):6468. https://doi.org/10.1038/s41598-019-42911-6
Masin M, Cuperova Z, Hojerova E, Salka I, Grossart HP, Koblizek M (2012) Distribution of aerobic anoxygenic phototrophic bacteria in glacial lakes of northern Europe. Aquat Microb Ecol 66(1):77–86. https://doi.org/10.3354/ame01558
Bibiloni-Isaksson J, Seymour JR, Ingleton T, van de Kamp JV, Bodrossy L, Brown MV (2016) Spatial and temporal variability of aerobic anoxygenic photoheterotrophic bacteria along the east coast of Australia. Environ Microbiol 18(12):4485–4500. https://doi.org/10.1111/1462-2920.13436
Rath KM, Rousk J (2015) Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol Biochem 81:108–123. https://doi.org/10.1016/j.soilbio.2014.11.001
Kopejtka K, Tomasch J, Zeng YH, Tichy M, Sorokin DY, Koblizek M (2017) Genomic Analysis of the Evolution of Phototrophy among Haloalkaliphilic Rhodobacterales. Genome Biol Evol 9(7):1950–1962. https://doi.org/10.1093/gbe/evx141
Drews G (1981) Rhodospirillum salexigens, spec. nov., an obligatory halophilic phototrophic bacterium. Arch Microbiol 130(4):325–327. https://doi.org/10.1007/BF00425949
Zhu D, Han R, Long Q, Gao X, **ng J, Shen G, Li Y, Wang R (2020) An evaluation of the core bacterial communities associated with hypersaline environments in the Qaidam Basin, China. Arch Microbiol 202(8):2093–2103. https://doi.org/10.1007/s00203-020-01927-7
Cytryn EJ, Sangurdekar DP, Streeter JG, Franck WL, Chang WS, Stacey G, Emerich DW, Joshi T, Xu D, Sadowsky MJ (2007) Transcriptional and Physiological Responses of Bradyrhizobium japonicum to Desiccation-Induced Stress. J Bacteriol 189(19):6751–6762. https://doi.org/10.1128/JB.00533-07
Seneviratne G, Indrasena IK (2006) Nitrogen fixation in lichens is important for improved rock weathering. J Biosci 31(5):639–643. https://doi.org/10.1007/Bf02708416
Maier S, Schmidt TSB, Zheng LJ, Peer T, Wagner V, Grube M (2014) Analyses of dryland biological soil crusts highlight lichens as an important regulator of microbial communities. Biodivers Conserv 23(7):1735–1755. https://doi.org/10.1007/s10531-014-0719-1
Mastrobuoni G, Irgang S, Pietzke M, Assmus HE, Wenzel M, Schulze WX, Kempa S (2012) Proteome dynamics and early salt stress response of the photosynthetic organism Chlamydomonas reinhardtii. BMC Genomics 13:215. https://doi.org/10.1186/1471-2164-13-215
Oren A (2014) The ecology of Dunaliella in high-salt environments. J Biol Res-Thessalon 21:21. https://doi.org/10.1186/s40709-014-0023-y
Darling RB, Friedmann EI, Broady PA (1987) HETEROCOCCUS ENDOLITHICUS SP. NOV. (XANTHOPHYCEAE) AND OTHER TERRESTRIAL HETEROCOCCUS SPECIES FROM ANTARCTICA: MORPHOLOGICAL CHANGES DURING LIFE HISTORY AND RESPONSE TO TEMPERATURE1. J Phycol 23(4):598–607. https://doi.org/10.1111/j.1529-8817.1987.tb04212.x
Hu CX, Zhang DL, Huang ZB, Liu YD (2003) The vertical microdistribution of cyanobacteria and green algae within desert crusts and the development of the algal crusts. Plant Soil 257(1):97–111. https://doi.org/10.1023/A:1026253307432
Tréguer P, Bowler C, Moriceau B, Dutkiewicz S, Gehlen M, Aumont O, Bittner L, Dugdale R, Finkel Z, Iudicone D, Jahn O, Guidi L, Lasbleiz M, Leblanc K, Levy M, Pondaven P (2018) Influence of diatom diversity on the ocean biological carbon pump. Nat Geosci 11(1):27–37. https://doi.org/10.1038/s41561-017-0028-x
Coste M, Boutry S, Tison-Rosebery J, Delmas F (2009) Improvements of the Biological Diatom Index (BDI): Description and efficiency of the new version (BDI-2006). Ecol Indic 9(4):621–650. https://doi.org/10.1016/j.ecolind.2008.06.003
Acknowledgments
We thank Wei Lin and Wensi Zhang from the Key Laboratory of Earth and Planetary Physics, Institute of Geology and Geophysics, Chinese Academy of Sciences for their assistance in soil sampling and would like to thank the editors and the reviewers for the valuable suggestions to improve the manuscript.
Declaration of Authors' Contribution
H.Y. and C.H. participated in the conception of the study, carried out the general design of experiments, collected and interpreted the data, and wrote the manuscript. H.Y. performed the experiments and analyzed the data. C.H. carried out critical revisions to the article for intellectual content. All authors have read and approved the final manuscript.
Funding
This work was funded by Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA17010502) and National Natural Science Foundation of China (No. 31900237).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, H., Hu, C. Soil Chemistry and Nutrients Influence the Distribution of Aerobic Anoxygenic Phototrophic Bacteria and Eukaryotic Phototrophic Microorganisms of Physical Soil Crusts at Different Elevations on the Tibetan Plateau. Microb Ecol 83, 100–113 (2022). https://doi.org/10.1007/s00248-021-01734-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01734-7