Abstract
Background
Liver Imaging Reporting and Data System (LI-RADS) has standardized the evaluation of hepatic lesions in adults at risk of develo** hepatocellular carcinoma (HCC). There is no accepted imaging algorithm for diagnosing HCC in the pediatric population.
Objective
The aim of our study was to evaluate the diagnostic accuracy and inter-rater reliability of LI-RADS version 2017 (v2017) for diagnosing HCC in a pediatric cohort.
Materials and methods
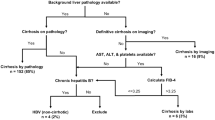
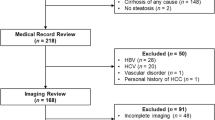
This retrospective, Institutional Review Board-approved study involved review of all abdominal dynamic contrast-enhanced imaging at a tertiary children’s hospital during a 10-year period, yielding 151 liver lesions in patients <18 years. Cases with active extrahepatic malignancy or an inadequate reference standard were excluded. Two readers independently evaluated all included hepatic lesions using LI-RADS criteria. Pathology and imaging follow-up were used as reference standards.
Results
A total of 41 lesions in 41 patients met criteria for evaluation (3 HCCs, 8 non-HCC malignancies, 30 benign lesions). A LI-RADS designation of definite HCC had high sensitivity (Reader 1/Reader 2: 100%, 95% confidence interval [CI] 31–100%) and high specificity (Reader 1: 84%, 95% CI: 68–93%; Reader 2: 97%, 95% CI: 85–100%) for predicting HCC. However, positive predictive value was only 33% (95% CI: 9–69%) and 75% (95% CI: 22–99%) for Reader 1 and Reader 2, respectively. For predicting any type of hepatic malignancy, a LI-RADS designation of definitely or likely malignant (i.e. not necessarily HCC) had a sensitivity of 100% (95% CI: 74–100%) and 90% (95% CI: 61–100%) for Reader 1 and Reader 2, respectively, and a negative predictive value (NPV) of 100% (95% CI: 81–100%) and 96% (95% CI: 83–99%) for Reader 1 and Reader 2, respectively. Interobserver agreement was substantial for the overall LI-RADS category (weighted κ=0.62; 95% CI: 0.38–0.86).
Conclusion
The positive predictive value of LI-RADS v2017 for diagnosing HCC was limited by the low frequency of HCC among pediatric patients. However, a LI-RADS designation of definitely or likely malignant had high sensitivity and NPV for any type of hepatic malignancy and may serve to direct clinical management by selecting patients for tissue sampling.







Similar content being viewed by others
References
Darbari A, Sabin KM, Shapiro CN, Schwarz KB (2003) Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 38:560–566
Schmid I, von Schweinitz D (2017) Pediatric hepatocellular carcinoma: challenges and solutions. J Hepatocell Carcinoma 4:15–21
Wang JD, Chang TK, Chen HC et al (2007) Pediatric liver tumors: initial presentation, image finding and outcome. Pediatr Int 49:491–496
Czauderna P (2002) Adult type vs. childhood hepatocellular carcinoma--are they the same or different lesions? Biology, natural history, prognosis, and treatment. Med Pediatr Oncol 39:519–523
Kassahun WT (2016) Contemporary management of fibrolamellar hepatocellular carcinoma: diagnosis, treatment, outcome, prognostic factors, and recent developments. World J Surg Oncol 14:151
Chan K, Fan S, Tam P et al (2002) Paediatric hepatoblastoma and hepatocellular carcinoma: retrospective study. Hong Kong Med J 8:13–17
Roberts LR, Sirlin CB, Zaiem F et al (2018) Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology 67:401–421
Mitchell DG, Bruix J, Sherman M, Sirlin CB (2015) LI-RADS (liver imaging reporting and data system): summary, discussion, and consensus of the LI-RADS Management working group and future directions. Hepatology 61:1056–1065
Tang A, Fowler KJ, Chernyak V et al (2018) LI-RADS and transplantation for hepatocellular carcinoma. Abdom Radiol 43:193–202
American College of Radiology (2017) CT/MRI LI-RADS v2017 core. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LIRADS_2017_Core.pdf?la=en. Accessed 30 Sept 2018
Brancatelli G, Federle MP, Grazioli L et al (2002) Benign regenerative nodules in Budd-Chiari syndrome and other vascular disorders of the liver: radiologic-pathologic and clinical correlation. Radiographics 22:847–862
Winston CB, Schwartz LH, Fong Y et al (1999) Hepatocellular carcinoma: MR imaging findings in cirrhotic livers and noncirrhotic livers. Radiology 210:75–79
Gaddikeri S, McNeeley MF, Wang CL et al (2014) Hepatocellular carcinoma in the noncirrhotic liver. Am J Roentgenol 203:W34–W47
Wang JD, Chang TK, Chen HC et al (2007) Pediatric liver tumors: initial presentation, image finding and outcome. Pediatr Int 49:491–496
Dubois J, Ditchfield M (2019) Neoplasia. In: Coley BD (ed) Caffey’s pediatric diagnostic imaging, 12th edn. Elsevier, Philadelphia, pp 838–850
Fleiss JL, Cohen J, Everitt BS (1969) Large sample standard errors of kappa and weighted kappa. Psychol Bull 72:323
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics:159–174
Mercaldo ND, Lau KF, Zhou XH (2007) Confidence intervals for predictive values with an emphasis to case–control studies. Stat Med 26:2170–2183
Fraum TJ, Tsai R, Rohe E et al (2017) Differentiation of hepatocellular carcinoma from other hepatic malignancies in patients at risk: diagnostic performance of the liver imaging reporting and data system version 2014. Radiology 286:158–172
Tang A, Bashir MR, Corwin MT et al (2017) Evidence supporting LI-RADS major features for CT-and MR imaging–based diagnosis of hepatocellular carcinoma: a systematic review. Radiology 286:29–48
Wald C, Russo MW, Heimbach JK et al (2013) New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology 266:376–382
Kelly D, Sharif K, Brown RM, Morland B (2015) Hepatocellular carcinoma in children. Clin Liver Dis 19:433–447
Sokollik C, Gupta A, Ling SC (2013) Hepatocellular carcinoma in children. In: Reau N, Poordad FF (eds) Primary liver cancer: surveillance, diagnosis and treatment. Humana Press/Springer, New York, pp 143–160
Fowler KJ, Tang A, Santillan C et al (2017) Interreader reliability of LI-RADS version 2014 algorithm and imaging features for diagnosis of hepatocellular carcinoma: a large international multireader study. Radiology 286:173–185
Acknowledgements
Dr. Ludwig receives salary support from the Training Opportunities in Translational Imaging Education and Research (TOP-TIER) Holden Thorp grant at Washington University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 62 kb)
Rights and permissions
About this article
Cite this article
Ludwig, D.R., Romberg, E.K., Fraum, T.J. et al. Diagnostic performance of Liver Imaging Reporting and Data System (LI-RADS) v2017 in predicting malignant liver lesions in pediatric patients: a preliminary study. Pediatr Radiol 49, 746–758 (2019). https://doi.org/10.1007/s00247-019-04358-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04358-9