Abstract
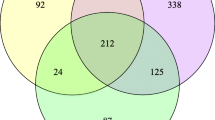
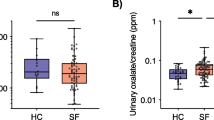
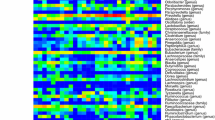
Individuals with urinary stone disease (USD) exhibit dysbiosis in the urinary tract and the loss of Lactobacillus that promote urinary tract health. However, the microbial metabolic functions that differentiate individuals with USD from healthy individuals are unknown. The objective of the current study was to determine the microbial functions across prokaryotic, viral, fungal, and protozoan domains that are associated with calcium oxalate (CaOx) stone formers through comparative shotgun metagenomics of midstream, voided urine samples for a small number of patients (n = 5 CaOx stone formers, n = 5 healthy controls). Results revealed that CaOx stone formers had reduced levels of genes associated with oxalate metabolism, as well as transmembrane transport, proteolysis, and oxidation–reduction processes. From 17 draft genomes extracted from the data and > 42,000 full length reference genomes, genes enriched in the Control group mapped overwhelming to Lactobacillus crispatus and those associated with CaOx mapped to Pseudomonas aeruginosa and Burkholderia sp. The microbial functions that differentiated the clinical cohorts are associated with known mechanisms of stone formation. While the prokaryotes most differentiated the CaOx and Control groups, a diverse, trans-domain microbiome was apparent. While our sample numbers were small, results corroborate previous studies and suggest specific microbial metabolic pathways in the urinary tract that modulate stone formation. Future studies that target these metabolic pathways as well as the influence of viruses, fungi, and protozoa on urinary tract physiology is warranted.








Similar content being viewed by others
Data availability
Raw sequence reads and metadata are available at the Sequence Read Archive under BioProject # PRJNA718167. Constructed genomes are available under BioProject #PRJNA717274.
Abbreviations
- USD:
-
Urinary stone disease
- CaOx:
-
Calcium oxalate
References
Ziemba JB, Matlaga BR (2017) Epidemiology and economics of nephrolithiasis. Investigative and clinical urology 58:299–306
Saigal CS, Joyce G, Timilsina AR, Project UDiA (2005) Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int 68:1808–1814
Batagello CM, Manoj; Miller, Aaron W. (2018) Urolithiasis: a case of missing microbes? J Endourol 32(11):995–1005
Zampini A, Nguyen AH, Rose E, Monga M, Miller AW (2019) Defining dysbiosis in patients with urolithiasis. Sci Rep 9:5425
Tasian GE, Thomas J, Goldfarb David S, Copelovitch L, Gerber Jeffrey S, Qufei Wu, Denburg Michelle R (2018) Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 29(6):1731–1740
Ferraro PM, Curhan GC, Gambaro G, Taylor EN (2019) Antibiotic use and risk of incident kidney stones in female nurses. Am J Kidney Dis 74:736–741
Dornbier RA, Bajic P, Van Kuiken M et al (2019) The microbiome of calcium-based urinary stones. Urolithiasis. 48(3):191–199
Sivaguru M, Saw JJ, Williams JC et al (2018) Geobiology reveals how human kidney stones dissolve in vivo. Sci Rep 8:13731
Wolfe AJ, Brubaker L (2015) “Sterile urine” and the presence of bacteria. Eur Urol 68:173
Liu F, Zhang N, Jiang P et al (2020) Characteristics of the urinary microbiome in kidney stone patients with hypertension. J Transl Med 18:1–13
**e J, Huang J-s, Huang X-j et al (2020) Profiling the urinary microbiome in men with calcium-based kidney stones. BMC Microbiol 20:1–10
Rani A, Ranjan R, McGee HS et al (2017) Urinary microbiome of kidney transplant patients reveals dysbiosis with potential for antibiotic resistance. Transl Res 181:59–70
Garretto A, Thomas-White K, Wolfe AJ, Putonti C (2018) Detecting viral genomes in the female urinary microbiome. J Gen Virol 99:1141
Bushnell B, Rood J, Singer E (2017) BBMerge–accurate paired shotgun read merging via overlap. PLoS ONE 12:e0185056
Li H (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. 1–3. ar**v preprint ar**v:1303.3997.
Nurk S, Meleshko D, Korobeynikov A, Pevzner PA (2017) metaSPAdes: a new versatile metagenomic assembler. Genome Res 27:824–834
Seemann T (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069
Fu L, Niu B, Zhu Z, Wu S, Li W (2012) CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28:3150–3152
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:1
Oksanen J, Blanchet FG, Kindt R, et al. (2010) Vegan: community ecology package. R package version 1.17–4. http://cran.r-project. org. Acesso em. 23:2010.
Miller IJ, Rees ER, Ross J et al (2019) Autometa: automated extraction of microbial genomes from individual shotgun metagenomes. Nucleic Acids Res 47:e57–e57
Chen K-T, Liu C-L, Huang S-H et al (2018) CSAR: a contig scaffolding tool using algebraic rearrangements. Bioinformatics 34:109–111
Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, Birol I (2009) ABySS: a parallel assembler for short read sequence data. Genome Res 19:1117–1123
Asnicar F, Thomas AM, Beghini F et al (2020) Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat Commun 11:1–10
Holmes RP, Assimos DG (2004) The impact of dietary oxalate on kidney stone formation. Urol Res 32:311–316
Tang J, McFann K, Chonchol M (2012) Dietary zinc intake and kidney stone formation: evaluation of NHANES III. Am J Nephrol 36:549–553
Chi T, Kim MS, Lang S et al (2015) A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS ONE 10:e0124150
Reddy ST, Wang C-Y, Sakhaee K, Brinkley L, Pak CY (2002) Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 40:265–274
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189:803–811
Khan SR (2014) Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol 3:256
Grin PM, Kowalewska PM, Alhazzan W, Fox-Robichaud AE (2013) Lactobacillus for preventing recurrent urinary tract infections in women: meta-analysis. Can J Urol 20:6607–6614
Nerurkar A, Solanky P, Naik SS (2012) Bacterial pathogens in urinary tract infection and antibiotic susceptibility pattern. J Pharm Biomed Sci 21:1–3.
Atilla Aridoğan I, Ilkit M, Izol V, Ates A (2005) Malassezia and Candida colonisation on glans penis of circumcised men. Mycoses 48:352–356
Fonseca F, Lanna M, Campos M et al (1998) Morphological and molecular characterization of the poxvirus BeAn 58058. Adv Virol 143:1171–1186
Goolam Mahomed T, Peters R, Allam M et al (2021) Lung microbiome of stable and exacerbated COPD patients in Tshwane South Africa. Sci Rep 11:1–12
van de Vossenberg J, Hoiting Y, Kamara AK et al (2021) Double-Stranded DNA virus assemblages in groundwater in three informal urban settlements in sub-Saharan Africa differ from each other. ACS ES&T Water 1:1992–2000
Mollerup S, Mikkelsen LH, Hansen AJ, Heegaard S (2019) High-throughput sequencing reveals no viral pathogens in eight cases of ocular adnexal extranodal marginal zone B-cell lymphoma. Exp Eye Res 185:107677
Isaac R, Reis FCG, Ying W, Olefsky JM (2021) Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab 33:1744–1762
Funding
NK was supported in part by the Urology Care Foundation Research Scholar Award Program and Endourological Society/Raju Thomas M.D. Award. Funding was also provided by NIH/NIDDK Grant 1R01DK121689-01A1 to AM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interests to report.
Ethics approval
The questionnaire and methodology for this study was approved by the Institutional Review Board of Cleveland Clinic (IRB# 16-643).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
240_2022_1314_MOESM4_ESM.xlsx
Table S2 Microbial genes significantly different between the CaOx and Controls. Listed are the log2 fold change, p value and adjusted p value, group enriched, which genome the genes mapped to either the de novo or reference genomes, and the complete Gene Ontology annotation of the genes.
Rights and permissions
About this article
Cite this article
Kachroo, N., Monga, M. & Miller, A.W. Comparative functional analysis of the urinary tract microbiome for individuals with or without calcium oxalate calculi. Urolithiasis 50, 303–317 (2022). https://doi.org/10.1007/s00240-022-01314-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-022-01314-5