Abstract
Objective
To systematically evaluate the efficacy of subcutaneous tocilizumab in the treatment of patients with severe COVID-19 and provide evidence for the rational use of subcutaneous tocilizumab in patients with severe COVID-19.
Methods
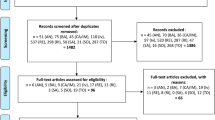
This meta-analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We searched the Cochrane Library, PubMed, Embase, CNKI, SinoMed, and Wanfang Medical Network electronic databases up to 11 January 2023 to identify relevant studies. To obtain the most recent clinical studies of subcutaneous injection of tocilizumab for the treatment of patients with severe COVID-19, we also searched the preprint platforms medRxiv and China**v. Furthermore, we searched ClinicalTrials.gov for relevant unpublished studies. The studies were screened based on the PICOS principle. The included studies were classified and evaluated for quality based on research type. The RevMan 5.3 software was used to conduct the meta-analysis, and a descriptive analysis was performed to examine relevant outcome indicators.
Results
Five observational studies were obtained, involving a total of 498 patients (240 patients in the subcutaneous injection group and 258 patients in the intravenous injection group). All of the studies were of the highest quality. The meta-analysis of the included studies revealed that the mortality rate of patients who received subcutaneous tocilizumab to treat COVID-19 was not significantly higher than that of the intravenous injection group [23.3% (45/193) vs. 18.4% (39/212), RD = 0.06, 95% CI = − 0.01 ~ 0.13, P = 0.11]. Furthermore, there was no significant difference in the proportion of patients requiring mechanical ventilation between the two groups [24.5% (35/143) vs. 22% (35/159), RD = 0.03, 95% CI = − 0.07 ~ 0.12, P = 0.56].
Conclusions
The meta-analyses do not provide evidence that subcutaneous and intravenous tocilizumab formulations for the treatment of severe COVID-19 infection differ regarding their effectiveness. Considering that the meta-analyses cannot replace an appropriately powered non-inferiority study, subcutaneous formulations still need to be used with caution and only when intravenous formulations are in short supply. At present, there is a lack of randomized controlled trials of subcutaneous injection of tocilizumab for the treatment of severe COVID-19, and more clinical research should be conducted.



Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
References
Liu J, Li S, Liu J et al (2020) Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55:102763. https://doi.org/10.1016/j.ebiom.2020.102763
Chen G, Wu D, Guo W et al (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130(5):2620–2629. https://doi.org/10.1172/JCI137244
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Behrens EM, Koretzky GA (2017) Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol 69(6):1135–1143. https://doi.org/10.1002/art.40071
Mehta P, McAuley DF, Brown M et al (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229):1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0
Liu B, Li M, Zhou Z et al (2020) Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun 111:102452. https://doi.org/10.1016/j.jaut.2020.102452
Henry BM, De Oliveira M, Benoit S et al (2020) Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 58(7):1021–1028. https://doi.org/10.1515/cclm-2020-0369
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10):a016295. https://doi.org/10.1101/cshperspect.a016295
Mastroianni A, Greco S, Apuzzo G et al (2020) Subcutaneous tocilizumab treatment in patients with severe COVID-19-related cytokine release syndrome: an observational cohort study. EClinicalMedicine 1(24):100410. https://doi.org/10.1016/j.eclinm.2020.100410
Potere N, Di Nisio M, Rizzo G et al (2020) Low-dose subcutaneous tocilizumab to prevent disease progression in patients with moderate COVID-19 pneumonia and hyperinflammation. Int J Infect Dis 100:421–424. https://doi.org/10.1016/j.ijid.2020.07.078
Higgins JPT, Green S. Cochrane handbook for systematic review of interventions 6.3 [updated August 2022] [J/OL]. (2011–03–10) [2020–07–21]. http://www.cochrane-handbook.org
Slim K, Nini E, Forestier D et al (2003) Methodological index for nonrandomized studies(minors): development and validation of a new instrument. ANZ J Surg 73(9):712–716
Wells GA, Shea B, O’Connell D et al. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses [EB/OL] (2015–07–21) [2020–6–1]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
De Rossi N, Scarpazza C, Filippini C et al (2020) Early use of low dose tocilizumab in patients with COVID-19: a retrospective cohort study with a complete follow-up. EClinicalMedicine 25:100459. https://doi.org/10.1016/j.eclinm.2020.100459
Guaraldi G, Meschiari M, Cozzi-Lepri A et al (2020) Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2(8):e474–e484. https://doi.org/10.1016/S2665-9913(20)30173-9
Kaminski MA, Sunny S, Balabayova K et al (2020) Tocilizumab therapy for COVID-19: a comparison of subcutaneous and intravenous therapies. Int J Infect Dis 101:59–64. https://doi.org/10.1016/j.ijid.2020.09.1447
Menzella F, Fontana M, Salvarani C et al (2020) Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Crit Care 24(1):589. https://doi.org/10.1186/s13054-020-03306-6
Sciascia S, Aprà F, Baffa A et al (2020) Pilot prospective open, single-arm multicenter study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol 38(3):529–532
Zhang X, Georgy A, Rowell L (2013) Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects. Int J Clin Pharmacol Ther 51(6):443–455. https://doi.org/10.5414/CP201819
Navas N, Hermosilla J, Torrente-López A et al (2020) Use of subcutaneous tocilizumab to prepare intravenous solutions for COVID-19 emergency shortage: comparative analytical study of physicochemical quality attributes. J Pharm Anal 10(6):532–545. https://doi.org/10.1016/j.jpha.2020.06.003
Malekzadeh R, Abedini A, Mohsenpour B et al (2020) Subcutaneous tocilizumab in adults with severe and critical COVID-19: a prospective open-label uncontrolled multicenter trial. Int Immunopharmacol 89(Pt B):107102. https://doi.org/10.1016/j.intimp.2020.107102
Author information
Authors and Affiliations
Contributions
Y.L contributed to the study conception and design and edited the manuscript. Y.L and Xj.Z analyzed the data and wrote the first draft of the manuscript. Y.L, Xj.Z, and Xx.L collected the data. All authors commented on final versions of the manuscript and revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study is deemed exempt to receive ethical approval.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Li, X. & Zheng, X. Effect of subcutaneous vs. intravenous tocilizumab in patients with severe COVID-19: a systematic review. Eur J Clin Pharmacol (2024). https://doi.org/10.1007/s00228-024-03719-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00228-024-03719-0