Abstract
Background
Glycyrrhizin is a major ingredient of licorice which is widely used in the treatment of various diseases such as chronic hepatitis. Licorice or glycyrrhizin has been shown to alter the activity of CYP3A in rodents. The influence of glycyrrhizin on CYP3A has not been elucidated in humans.
Objective
To investigate the effects of repeated glycyrrhizin ingestion on the oral pharmacokinetics of midazolam, a probe drug for CYP3A activity in humans.
Methods
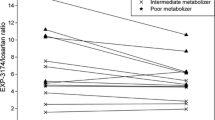
Sixteen healthy adult male subjects were enrolled in a two-phase randomized crossover design. In each phase the volunteers received placebo or glycyrrhizin for 14 days. On the 15th day, midazolam was administered and blood samples were obtained to determine midazolam plasma concentrations. Bioequivalence was assessed by determining geometric mean ratios (GMRs) and 90% confidence intervals (90% CI).
Results
The geometric mean (geometric coefficient of variation) for the \( {\hbox{AU}}{{\hbox{C}}_{0 - \infty }} \) of midazolam in the placebo group was 196.4 ng·h/ml (30.3%) and after glycyrrhizin treatment, 151.3 ng·h/ml (34.7%). The GMRs and 90% CI for \( {\hbox{AU}}{{\hbox{C}}_{0 - \infty }} \) and Cmax of midazolam in the presence/absence of glycyrrhizin were 0.77 (0.70, 0.89) and 0.83 (0.74, 1.01), respectively. The 90% CI for \( {\hbox{AU}}{{\hbox{C}}_{0 - \infty }} \) and Cmax for the GMR of glycyrrhizin over placebo were both out of the no-effect boundaries of 0.80–1.25.
Conclusions
Administration of glycyrrhizin resulted in a modest induction of CYP3A that was clinically relevant according to the bioequivalence analysis.


Similar content being viewed by others
References
Eisenbrand G (2006) Glycyrrhizin. Mol Nutr Food Res 50:1087–1088
van Rossum TG, Vulto AG, de Man RA, Brouwer JT, Schalm SW (1998) Review article: glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol Ther 12:199–205
Hattori T, Ikematsu S, Koito A et al (1989) Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antiviral Res 11:255–261
Isbrucker RA, Burdock GA (2006) Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol 46:167–192
Arase Y, Ikeda K, Murashima N et al (1997) The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 79(8):1494–1500
Paolini M, Pozzetti L, Sapone A, Cantelli-Forti G (1998) Effect of licorice and glycyrrhizin on murine liver CYP-dependent monooxygenases. Life Sci 62:571–582
Paolini M, Barillari J, Broccoli M, Pozzetti L, Perocco P, Cantelli-Forti G (1999) Effect of liquorice and glycyrrhizin on rat liver carcinogen metabolizing enzymes. Cancer Lett 18(145):35–42
Bogaards JJ, Bertrand M, Jackson P et al (2000) Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica 30:1131–1152
Kolars JC, Lown KS, Schmiedlin-Ren P et al (1994) CYP3A gene expression in human gut epithelium. Pharmacogenetics 4:247–259
Prueksaritanont T, Gorham LM, Hochman JH, Tran LO, Vyas KP (1996) Comparative studies of drug-metabolizing enzymes in dog, monkey, and human small intestines, and in Caco-2 cells. Drug Metab Dispos 24:634–642
Thummel KE, Shen DD, Podoll TD et al (1994) Use of midazolam as a human cytochrome P450, 3A probe. I. In vitro–in vivo correlations in liver transplant patients. J Pharmacol Exp Ther 271:549–556
Backman JT, Kivisto KT, Olkkola KT, Neuvonen PJ (1998) The area under the plasma concentration-time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur J Clin Pharmacol 54:53–58
Thummel KE, O’Shea D, Paine MF et al (1996) Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther 59:491–502
Paine MF, Shen DD, Kunze KL et al (1996) First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther 60:14–24
Moore LB, Parks DJ, Jones SA et al (2000) Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275:15122–15127
Moore LB, Maglich JM, McKee DD et al (2002) Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol 16:977–986
Sonoda J, Rosenfeld JM, Xu L, Evans RM, **e W (2003) A nuclear receptor-mediated xenobiotic response and its implication in drug metabolism and host protection. Curr Drug Metab 4:59–72
Wang H, LeCluyse EL (2003) Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet 42:1331–1357
Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA (1998) The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016–1023
Goodwin B, Redinbo MR, Kliewer SA (2002) Regulation of cyp3a gene transcription by the pregnane x receptor. Annu Rev Pharmacol Toxicol 42:1–23
Mu Y, Zhang J, Zhang S et al (2006) Traditional Chinese medicines wu wei zi (Schisandra chinensis Baill) and gan cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J Pharmacol Exp Ther 316:1369–1377
Takeda S, Ishthara K, Wakui Y et al (1996) Bioavailability study of glycyrrhetic acid after oral administration of glycyrrhizin in rats; relevance to the intestinal bacterial hydrolysis. J Pharm Pharmacol 48:902–905
Huang SM, Temple R, Throckmorton DC, Lesko LJ (2007) Drug interaction studies: study design, data analysis, and implications for dosing and labeling. Clin Pharmacol Ther 81:298–304
Conflict of Interest
The authors declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Funding source
This work was supported by the National Scientific Foundation of China (30472054, 30801421, 30901834), Huge Project to Boost Chinese Drug Development, (2009ZX09501-032), Fund by State Key Laboratory of Drug Research (SIMM0812KF-01) and Research Fund for the Doctoral Program of Higher Education of China (20070533002).
Rights and permissions
About this article
Cite this article
Tu, JH., He, YJ., Chen, Y. et al. Effect of glycyrrhizin on the activity of CYP3A enzyme in humans. Eur J Clin Pharmacol 66, 805–810 (2010). https://doi.org/10.1007/s00228-010-0814-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0814-5