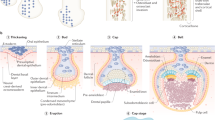
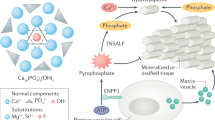
Abstract
Inorganic phosphate is a vital constituent of cells and cell membranes, body fluids, and hard tissues. It is a major intracellular divalent anion, participates in many genetic, energy and intermediary metabolic pathways, and is important for bone health. Although we usually think of phosphate mostly in terms of its level in the serum, it is needed for many biological and structural functions of the body. Availability of adequate calcium and inorganic phosphate in the right proportions at the right place is essential for proper acquisition, biomineralization, and maintenance of mass and strength of the skeleton. The three specialized mineralized tissues, bones, teeth, and ossicles, differ from all other tissues in the human body because of their unique ability to mineralize, and the degree and process of mineralization in these tissues also differ to suit the specific functions: locomotion, chewing, and hearing, respectively. Biomineralization is a dynamic, complex, and lifelong process by which precipitations of inorganic calcium and inorganic phosphate divalent ions form biological hard tissues. Understanding the biomineralization process is important for the management of diseases caused by both defective and abnormal mineralization. Hypophosphatemia results in mineralization defects and osteomalacia, and hyperphosphatemia is implicated in abnormal excess calcification and/or ossification, but the exact mechanisms underlying these processes are not fully understood. In this review, we summarize available evidence on the role of phosphate in biomineralization. Other manuscripts in this issue of the journal deal with other relevant aspects of phosphate homeostasis, phosphate signaling and sensing, and disorders resulting from hypo- and hyperphosphatemic states.


Similar content being viewed by others
References
Chande S, Bergwitz C (2018) Role of phosphate sensing in bone and mineral metabolism. Nat Rev Endocrinol 14(11):637–655. https://doi.org/10.1038/s41574-018-0076-3
Goretti Penido M, Alon US (2012) Phosphate homeostasis and its role in bone health. Pediatr Nephrol (Berlin, Germany) 27(11):2039–2048. https://doi.org/10.1007/s00467-012-2175-z
Michigami T, Kawai M, Yamazaki M, Ozono K (2018) Phosphate as a signaling molecule and its sensing mechanism. Physiol Rev 98(4):2317–2348. https://doi.org/10.1152/physrev.00022.2017
Bonjour JP (2011) Calcium and phosphate: a duet of ions playing for bone health. J Am Coll Nutr 30(5 Suppl 1):438s–448s. https://doi.org/10.1080/07315724.2011.10719988
Tenenhouse HS (2007) Phosphate transport: molecular basis, regulation and pathophysiology. J Steroid Biochem Mol Biol 103(3–5):572–577. https://doi.org/10.1016/j.jsbmb.2006.12.090
Sapio L, Naviglio S (2015) Inorganic phosphate in the development and treatment of cancer: a Janus Bifrons? World J Clin Oncol 6(6):198–201. https://doi.org/10.5306/wjco.v6.i6.198
Michigami T (2019) Skeletal mineralization: mechanisms and diseases. Ann Pediatr Endocrinol Metab 24(4):213–219. https://doi.org/10.6065/apem.2019.24.4.213
Kawasaki K, Buchanan AV, Weiss KM (2009) Biomineralization in humans: making the hard choices in life. Annu Rev Genet 43:119–142. https://doi.org/10.1146/annurev-genet-102108-134242
Abou Neel EA, Aljabo A, Strange A, Ibrahim S, Coathup M, Young AM, Bozec L, Mudera V (2016) Demineralization–remineralization dynamics in teeth and bone. Int J Nanomed 11:4743–4763. https://doi.org/10.2147/ijn.S107624
Bhan A, Qiu SJ, Rao SD (2018) Bone histomorphometry in the evaluation of osteomalacia. Bone Rep 8:124–135
Basha B, Rao DS, Han ZH, Parfitt AM (2000) Osteomalacia due to vitamin D depletion: a neglected consequence of intestinal malabsorption. Am J Med 108:296–300
Minisola S, Peacock M, Fukumoto S, Cipriani C, Pepe J, Tella SH, Collins MT (2017) Tumour-induced osteomalacia. Nat Rev Dis Primers 3:17044. https://doi.org/10.1038/nrdp.2017.44
Claes KJ, Viaene L, Heye S, Meijers B, d'Haese P, Evenepoel P (2013) Sclerostin: another vascular calcification inhibitor? J Clin Endocrinol Metab 98(8):3221–3228. https://doi.org/10.1210/jc.2013-1521
Brandenburg VM, Kramann R, Koos R, Kruger T, Schurgers L, Muhlenbruch G, Hubner S, Gladziwa U, Drechsler C, Ketteler M (2013) Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol 14:219. https://doi.org/10.1186/1471-2369-14-219
Komarova SV, Safranek L, Gopalakrishnan J, Ou MJ, McKee MD, Murshed M, Rauch F, Zuhr E (2015) Mathematical model for bone mineralization. Front Cell Dev Biol 3:51. https://doi.org/10.3389/fcell.2015.00051
Hughes EAB, Robinson TE, Bassett DB, Cox SC, Grover LM (2019) Critical and diverse roles of phosphates in human bone formation. J Mater Chem B 7(47):7460–7470. https://doi.org/10.1039/C9TB02011J
Reznikov N, Shahar R, Weiner S (2014) Bone hierarchical structure in three dimensions. Acta Biomater 10(9):3815–3826. https://doi.org/10.1016/j.actbio.2014.05.024
Dillon S, Staines KA, Millan JL, Farquharson C (2019) How to build a bone: PHOSPHO1, biomineralization, and beyond. JBMR Plus 3(7):e10202. https://doi.org/10.1002/jbm4.10202
Orimo H, Shimada T (2008) The role of tissue-nonspecific alkaline phosphatase in the phosphate-induced activation of alkaline phosphatase and mineralization in SaOS-2 human osteoblast-like cells. Mol Cell Biochem 315(1–2):51–60. https://doi.org/10.1007/s11010-008-9788-3
Halling Linder C, Ek-Rylander B, Krumpel M, Norgard M, Narisawa S, Millan JL, Andersson G, Magnusson P (2017) Bone alkaline phosphatase and tartrate-resistant acid phosphatase: potential co-regulators of bone mineralization. Calcif Tissue Int 101(1):92–101. https://doi.org/10.1007/s00223-017-0259-2
Florenzano P, Cipriani C, Roszko KL, Fukumoto S, Collins MT, Minisola S, Pepe J (2020) Approach to patients with hypophosphataemia. Lancet Diabetes Endocrinol 8(2):163–174. https://doi.org/10.1016/S2213-8587(19)30426-7
Hasegawa T (2018) Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem Cell Biol 149(4):289–304. https://doi.org/10.1007/s00418-018-1646-0
Chaudhary SC, Kuzynski M, Bottini M, Beniash E, Dokland T, Mobley CG, Yadav MC, Poliard A, Kellermann O, Millan JL, Napierala D (2016) Phosphate induces formation of matrix vesicles during odontoblast-initiated mineralization in vitro. Matrix Biol J Int Soc Matrix Biol 52–54:284–300. https://doi.org/10.1016/j.matbio.2016.02.003
Zhang J, Wang L, Putnis CV (2019) Underlying role of brushite in pathological mineralization of hydroxyapatite. J Phys Chem B 123(13):2874–2881. https://doi.org/10.1021/acs.jpcb.9b00728
Cui L, Houston DA, Farquharson C, MacRae VE (2016) Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 87:147–158. https://doi.org/10.1016/j.bone.2016.04.007
Wergedal JE, Baylink DJ (1974) Electron microprobe measurements of bone mineralization rate in vivo. Am J Physiol 226(2):345–352. https://doi.org/10.1152/ajplegacy.1974.226.2.345
Zhang X, Huang X, Ma M (2017) Role of phosphorylation of phosvitin in the phase transformation of mineralization. Int J Biol Macromol 101:712–718. https://doi.org/10.1016/j.ijbiomac.2017.03.158
Poundarik AA, Boskey A, Gundberg C, Vashishth D (2018) Biomolecular regulation, composition and nanoarchitecture of bone mineral. Sci Rep 8(1):1191. https://doi.org/10.1038/s41598-018-19253-w
Parfitt AM (1992) Human bone mineralization studied by in vivo tetracycline labeling: application to the pathophysiology of osteomalacia. In: Excerpta medica international congress, 4th international symposium on chemistry and biology of mineralized tissues, Coronado Peninsula, California, pp 465–474
Ben Shoham A, Rot C, Stern T, Krief S, Akiva A, Dadosh T, Sabany H, Lu Y, Kadler KE, Zelzer E (2016) Deposition of collagen type I onto skeletal endothelium reveals a new role for blood vessels in regulating bone morphology. Development 143(21):3933–3943. https://doi.org/10.1242/dev.139253
Velentzas C, Meindok H, Oreopoulos DG, Meema HE, Rabinovich S, Jones M, Sutton D, Rapoport A, deVeber GA (1978) Visceral calcification and the CaXP product. Adv Exp Med Biol 103:195–201. https://doi.org/10.1007/978-1-4684-7758-0_21
Erben RG, Andrukhova O (2017) FGF23-Klotho signaling axis in the kidney. Bone 100:62–68. https://doi.org/10.1016/j.bone.2016.09.010
Tatsumi S, Miyagawa A, Kaneko I, Shiozaki Y, Segawa H, Miyamoto K (2016) Regulation of renal phosphate handling: inter-organ communication in health and disease. J Bone Miner Metab 34(1):1–10. https://doi.org/10.1007/s00774-015-0705-z
Michigami T, Ozono K (2019) Roles of phosphate in skeleton. Front Endocrinol 10:180. https://doi.org/10.3389/fendo.2019.00180
Liu ES, Zalutskaya A, Chae BT, Zhu ED, Gori F, Demay MB (2014) Phosphate interacts with PTHrP to regulate endochondral bone formation. Endocrinology 155(10):3750–3756. https://doi.org/10.1210/en.2014-1315
Miedlich SU, Zalutskaya A, Zhu ED, Demay MB (2010) Phosphate-induced apoptosis of hypertrophic chondrocytes is associated with a decrease in mitochondrial membrane potential and is dependent upon Erk1/2 phosphorylation. J Biol Chem 285(24):18270–18275. https://doi.org/10.1074/jbc.M109.098616
Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F (2000) Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci 113(Pt 1):59–69
Sabbagh Y, Carpenter TO, Demay MB (2005) Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA 102(27):9637–9642. https://doi.org/10.1073/pnas.0502249102
Donohue MM, Demay MB (2002) Rickets in VDR null mice is secondary to decreased apoptosis of hypertrophic chondrocytes. Endocrinology 143(9):3691–3694. https://doi.org/10.1210/en.2002-220454
Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423(6937):332–336. https://doi.org/10.1038/nature01657
Bhan A, Rao AD, Bhadada SK, Rao DS (2020) Rickets and osteomalacia. In: Melmed S, Auchus RJ, Goldfine AB, Koenig RJ, Rosen CJ (eds) Williams textbook of endocrinology, 14th edn. Elsevier, Philadelphia, pp 1298–1317
Tiosano D, Hochberg Z (2009) Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab 27(4):392–401. https://doi.org/10.1007/s00774-009-0079-1
Shroff R (2013) Phosphate is a vascular toxin. Pediatr Nephrol (Berlin, Germany) 28(4):583–593. https://doi.org/10.1007/s00467-012-2347-x
Yamada S, Giachelli CM (2017) Vascular calcification in CKD-MBD: roles for phosphate, FGF23, and Klotho. Bone 100:87–93. https://doi.org/10.1016/j.bone.2016.11.012
Floege J (2004) When man turns to stone: extraosseous calcification in uremic patients. Kidney Int 65(6):2447–2462. https://doi.org/10.1111/j.1523-1755.2004.00664.x
Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM, Towler DA (2011) Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res 109(6):697–711. https://doi.org/10.1161/CIRCRESAHA.110.234914
Alfrey AC, Solomons CC, Ciricillo J, Miller NL (1976) Extraosseous calcification. Evidence for abnormal pyrophosphate metabolism in uremia. J Clin Investig 57(3):692–699. https://doi.org/10.1172/JCI108326
Giachelli CM (2009) The emerging role of phosphate in vascular calcification. Kidney Int 75(9):890–897. https://doi.org/10.1038/ki.2008.644
Paloian NJ, Giachelli CM (2014) A current understanding of vascular calcification in CKD. Am J Physiol Ren Physiol 307(8):F891–F900. https://doi.org/10.1152/ajprenal.00163.2014
Pai AS, Giachelli CM (2010) Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol JASN 21(10):1637–1640. https://doi.org/10.1681/asn.2010040349
Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM (2004) Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol JASN 15(11):2857–2867. https://doi.org/10.1097/01.Asn.0000141960.01035.28
Hosaka N, Mizobuchi M, Ogata H, Kumata C, Kondo F, Koiwa F, Kinugasa E, Akizawa T (2009) Elastin degradation accelerates phosphate-induced mineralization of vascular smooth muscle cells. Calcif Tissue Int 85(6):523–529. https://doi.org/10.1007/s00223-009-9297-8
Barvencik F, Beil FT, Gebauer M, Busse B, Koehne T, Seitz S, Zustin J, Pogoda P, Schinke T, Amling M (2011) Skeletal mineralization defects in adult hypophosphatasia—a clinical and histological analysis. Osteoporos Int 22(10):2667–2675. https://doi.org/10.1007/s00198-011-1528-y
Singer FR (2016) Bone quality in Paget’s disease of bone. Curr Osteoporos Rep 14(2):39–42. https://doi.org/10.1007/s11914-016-0303-6
Corsi A, Collins MT, Riminucci M, Howell PG, Boyde A, Robey PG, Bianco P (2003) Osteomalacic and hyperparathyroid changes in fibrous dysplasia of bone: core biopsy studies and clinical correlations. J Bone Miner Res 18(7):1235–1246. https://doi.org/10.1359/jbmr.2003.18.7.1235
Bhadada SK, Dhiman V, Mukherjee S, Aggarwal S, Bal A, Sukumar SP, Sood A, Sharma DC, Khandelwal N, Bhansali A, Van Hul W, Rao SD (2017) Fibrogenesis imperfecta ossium and response to human growth hormone: a potential therapy. J Clin Endocrinol Metab 102(5):1750–1756. https://doi.org/10.1210/jc.2016-3055
Marini JC, Forlino A, Bachinger HP, Bishop NJ, Byers PH, Paepe A, Fassier F, Fratzl-Zelman N, Kozloff KM, Krakow D, Montpetit K, Semler O (2017) Osteogenesis imperfecta. Nat Rev Dis Primers 3:17052. https://doi.org/10.1038/nrdp.2017.52
Hoppe E, Masson C, Laffitte A, Chappard D, Audran M (2012) Osteomalacia in a patient with Paget’s bone disease treated with long-term etidronate. Morphologie: bulletin de l'Association des anatomistes 96(313):40–43. https://doi.org/10.1016/j.morpho.2012.08.001
Balena R, Kleerekoper M, Foldes JA, Shih MS, Rao DS, Schober HC, Parfitt AM (1998) Effects of different regimens of sodium fluoride treatment for osteoporosis on the structure, remodeling and mineralization of bone. Osteoporos Int 8:428–435
Teotia M, Teotia SP, Singh KP (1998) Endemic chronic fluoride toxicity and dietary calcium deficiency interaction syndromes of metabolic bone disease and deformities in India. Indian J Pediatr 65:371–381
Quarles LD, Dennis VW, Gitelman HJ, Harrelson JM, Drezner MK (1985) Aluminum deposition at the osteoid–bone interface. An epiphenomenon of the osteomalacic state in vitamin D-deficient dogs. J Clin Invest 75(5):1441–1447. https://doi.org/10.1172/jci111846
Parfitt AM, Rao DS, Stanciu J, Villanueva AR (1986) Comparison of aluminum related with vitamin D related osteomalacia by tetracycline based bone histomorphometry. Adv Exp Med Biol 208:283–287
Matsushima S, Torii M, Ozaki K, Narama I (2003) Iron lactate-induced osteomalacia in association with osteoblast dynamics. Toxicol Pathol 31(6):646–654. https://doi.org/10.1080/01926230390241990
Parfitt AM, Qiu S, Rao DS (2004) The mineralization index—a new approach to the histomorphometric appraisal of osteomalacia. Bone 35:320–325
Chines A, Pacifici R (1990) Antacid and sucralfate-induced hypophosphatemic osteomalacia: a case report and review of the literature. Calcif Tissue Int 47(5):291–295
Fabbriciani G, de Socio GV, Massarotti M, Ceriani R, Marasini B (2011) Adefovir induced hypophosphatemic osteomalacia. Scand J Infect Dis 43(11–12):990–992. https://doi.org/10.3109/00365548.2011.581307
Mateo L, Holgado S, Marinoso ML, Perez-Andres R, Bonjoch A, Romeu J, Olive A (2016) Hypophosphatemic osteomalacia induced by tenofovir in HIV-infected patients. Clin Rheumatol 35(5):1271–1279. https://doi.org/10.1007/s10067-10014-12627-x
Sansone V, Maiorano E, Galluzzo A, Pascale V (2018) Calcific tendinopathy of the shoulder: clinical perspectives into the mechanisms, pathogenesis, and treatment. Orthop Res Rev 10:63–72. https://doi.org/10.2147/orr.S138225
Rosenthal AK, Ryan LM (2016) Calcium pyrophosphate deposition disease. N Engl J Med 374(26):2575–2584. https://doi.org/10.1056/NEJMra1511117
Taniwaki M, Kawamoto K, Yamasaki M, Funaishi K, Matsumoto Y, Matsumoto N, Ohashi N, Hattori N (2019) Severe metastatic pulmonary calcification. Am J Med 132(10):e733–e734. https://doi.org/10.1016/j.amjmed.2019.04.031
Mignemi NA, Yuasa M, Baker CE, Moore SN, Ihejirika RC, Oelsner WK, Wallace CS, Yoshii T, Okawa A, Revenko AS, MacLeod AR, Bhattacharjee G, Barnett JV, Schwartz HS, Degen JL, Flick MJ, Cates JM, Schoenecker JG (2017) Plasmin prevents dystrophic calcification after muscle injury. J Bone Miner Res 32(2):294–308. https://doi.org/10.1002/jbmr.2973
Lee DK, Eiferman RA (2006) Ocular calcifications in primary hyperparathyroidism. Arch Ophthalmol 124(1):136–137. https://doi.org/10.1001/archopht.124.1.136
Yashiro T, Okamoto T, Tanaka R, Ito K, Hara H, Yamashita T, Kanaji Y, Kodama T, Ito Y, Obara T et al (1991) Prevalence of chondrocalcinosis in patients with primary hyperparathyroidism in Japan. Endocrinol Jpn 38(5):457–464. https://doi.org/10.1507/endocrj1954.38.457
Klaassen-Broekema N, van Bijsterveld OP (1993) Limbal and corneal calcification in patients with chronic renal failure. Br J Ophthalmol 77(9):569–571. https://doi.org/10.1136/bjo.77.9.569
Braunstein EM, Menerey K, Martel W, Swartz R, Fox IH (1987) Radiologic features of a pyrophosphate-like arthropathy associated with long-term dialysis. Skeletal Radiol 16(6):437–441. https://doi.org/10.1007/bf00350536
Gaisne R, Pere M, Menoyo V, Hourmant M, Larmet-Burgeot D (2020) Calciphylaxis epidemiology, risk factors, treatment and survival among French chronic kidney disease patients: a case–control study. BMC Ephrology 21(1):63. https://doi.org/10.1186/s12882-020-01722-y
Lecoq AL, Brandi ML, Linglart A, Kamenicky P (2020) Management of X-linked hypophosphatemia in adults. Metabolism 103s:154049. https://doi.org/10.1016/j.metabol.2019.154049
Whyte MP, Amalnath SD, McAlister WH, McKee MD, Veis DJ, Huskey M, Duan S, Bijanki VN, Alur S, Mumm S (2020) Hypophosphatemic osteosclerosis, hyperostosis, and enthesopathy associated with novel homozygous mutations of DMP1 encoding dentin matrix protein 1 and SPP1 encoding osteopontin: the first digenic SIBLING protein osteopathy? Bone 132:115190. https://doi.org/10.1016/j.bone.2019.115190
Marcucci G, Cianferotti L, Brandi ML (2018) Clinical presentation and management of hypoparathyroidism. Best Pract Res Clin Endocrinol Metab 32(6):927–939. https://doi.org/10.1016/j.beem.2018.09.007
Goswami R, Sharma R, Sreenivas V, Gupta N, Ganapathy A, Das S (2012) Prevalence and progression of basal ganglia calcification and its pathogenic mechanism in patients with idiopathic hypoparathyroidism. Clin Endocrinol (Oxf) 77(2):200–206. https://doi.org/10.1111/j.1365-2265.2012.04353.x
Mufaddel AA, Al-Hassani GA (2014) Familial idiopathic basal ganglia calcification (Fahr’s disease). Neurosciences (Riyadh, Saudi Arabia) 19(3):171–177
Carr DR, Pootrakul L, Chen HZ, Chung CG (2019) Metastatic calcinosis cutis associated with a selective FGFR inhibitor. JAMA Dermatol 155(1):122–123. https://doi.org/10.1001/jamadermatol.2018.4070
Fathi I, Sakr M (2014) Review of tumoral calcinosis: a rare clinico-pathological entity. World J Clin Cases 2(9):409–414. https://doi.org/10.12998/wjcc.v2.i9.409
Yolcu YU, Wahood W, Goyal A, Alvi MA, Reeves RK, Qu W, Gerberi DJ, Bydon M (2020) Pharmacologic prophylaxis for heterotopic ossification following spinal cord injury: a systematic review and meta-analysis. Clin Neurol Neurosurg 193:105737. https://doi.org/10.1016/j.clineuro.2020.105737
Rao DS, Parfitt AM, Kleerekoper M, Pumo BS, Frame B (1985) Dissociation between the effects of endogenous parathyroid hormone on adenosine 3′,5′-monophosphate generation and phosphate reabsorption in hypocalcemia due to vitamin D depletion: an acquired disorder resembling pseudohypoparathyroidism type II. J Clin Endocrinol Metab 61(2):285–290. https://doi.org/10.1210/jcem-61-2-285
Bhadada SK, Palnitkar S, Qiu S, Parikh N, Talpos GB, Rao SD (2013) Deliberate total parathyroidectomy: a potentially novel therapy for tumor-induced hypophosphatemic osteomalacia. J Clin Endocrinol Metab 98(11):4273–4278. https://doi.org/10.1210/jc.2013-2705
Parfitt AM (1990) Osteomalacia and related disorders. In: Avioli LV, Krane SM (eds) Metabolic bone disease and clinically related disorders, vol 2. W.B. Saunders, Philadelphia, pp 329–396
Funding
The study was partly supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (AR062103).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sudhaker D. Rao and Sanjay Kumar Bhadada declare that they have no conflict of interest.
Ethical Approval
The study was conducted in accordance with the ethical standards of the institutional research committee of Henry Ford Hospital and the 1964 Helsinki Declaration and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhadada, S.K., Rao, S.D. Role of Phosphate in Biomineralization. Calcif Tissue Int 108, 32–40 (2021). https://doi.org/10.1007/s00223-020-00729-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00729-9