Abstract
Although blossom and honeydew honeys are produced from different nectar sources, it is very difficult to distinguish them. In this study, physicochemical and biochemical properties were compared to distinguish honeydew and blossom honeys obtained from different botanical sources. The honeys of pine (Pinus spp.) and oak (Quercus, spp.) were used as honeydew honey, and chestnut (Castania sativa L.) and highland honey were used as blossom honey. Turbidity, specific optical rotation (SOR) [α]20, electrical conductivity (EC), CIEL* a*b color parameters, and moisture and pH were assessed as physical parameters. Proline, invertase activity, sugar composition, total phenolic and flavonoid contents, and antioxidant capacity were used as biochemical properties. It was determined that SOR values were the most important parameters in distinguishing honeydew and blossom honeys. Turbidity stands out as a crucial factor in differentiating between pine and oak honeys. Except for chestnut honey, electrical conductivity, CIE color Lab values, and sugar profiles were identified as additional distinguishing factors among the tested honeys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Honey, a remarkable natural product crafted by honeybees, boasts a diverse composition and biologically active properties. Its rich, syrupy blend of carbohydrates showcases variability influenced by multiple factors: plant flora, geographical nuances, bee species, production time, and type. Honey is categorically segregated into two main subclasses, blossom, and honeydew honeys. The honey produced from the liquids collected from the flower nectar of honey plants is called blossom honey. Honeydew honey, on the other hand, originates from nectarless plants through distinct methods [1,2,3].
Two distinct varieties of honeydew honey exist. The initial type originates from sugary residues generated by diverse insects, notably pine lice feeding on plant sap, with pine honey serving as a prominent illustration. Pine (Pinus spp.) honey derives from secretions by Marchalina hellenica insects, aphids that reside within pine trees. This honey, produced in Mediterranean region countries, especially Turkey, does not crystallize easily [2, 4,5,6,7]. Visually, honey has a slightly compact appearance. However, the extent of this honey has not been measured anywhere. Other honeydew honey is produced from sugary substances that leak in the form of sweat according to the physiological needs of the trees [3]. This physiological condition, which is generally seen in most trees, is at a high level in oak trees (Quercus spp.). Although there are many species of oak trees, the Quercus frainetto is the most prone to honeydew production. Approximately 500 tons of oak honey are produced every year from the oak forests in the Kırklareli region, located in the Northwest region of Turkey. Honeydew honey production in the region depends on the climate, especially in the summer when there is little rain, the secretions leak from the leaves and fruits of the tree [5]. This dark honey is highly viscous and does not crystallize easily.
Blossom honeys are divided into two subclasses according to their nectar sources: forest honeys and herbaceous plant honeys. Since Türkiye has an important transition zone between the Asian, European, and African continents, it is home to a very rich honey plant. Herbaceous plant honeys such as myosotis, lavender, astragalus, thyme, black cumin, sunflower, ivy, euphorbia, cotton, etc., are produced as monofloral or heterofloral [8, 9]. These honeys, especially light-colored ones that can crystallize easily, are mostly preferred as edible honeys. Chestnut honey is a dark-colored forest honey and has high bioactive compounds, mostly polyphenols [5, 10]. Chestnut honey has different properties from many blossom honeys in terms of physicochemical properties, and significant differences are shown in honey codex [11]. Chestnut honey, which has a woody taste that burns the throat, is mostly used for medical applications such as upper respiratory tract diseases and asymptomatic treatments [11, 12]. Chestnut honey, produced mostly in the Black Sea Region, is a slightly blurry-looking and non-crystallized honey.
The biochemical and physicochemical properties of each species of honey vary in terms of both plant flora and production techniques. However, according to international honey codes, some different physicochemical properties of blossom honey, forest honey, and honeydew honey have been determined and added to the regulations. In this study, it was aimed to compare some important physicochemical and biochemical properties of honeydew and blossom honeys, especially turbidity, optical rotation, and phenolic composition values.
Materials and methods
Honey samples
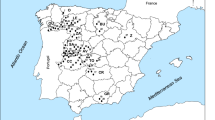
In the study, two different honeydew honey species and two different blossom honey species of samples were collected by the Turkish Beekeepers Association (TAB) in 2019 from Türkiye. Eight heterofloral blossom honey samples were collected by the highland of the Kars region, Northeastern Anatolia of Türkiye. Sixteen chestnut honeys were collected from the Zonguldak and Bursa regions of North Türkiye. Nine pine honey samples were collected from Muğla around the Aegean region. Nine oak honey samples were collected from the Kırklareli region, Northwestern region of Türkiye. Table 1 shows the geographical units of the regions where the samples were collected. The procedures to be applied to prepare the honey samples coming to the laboratory for analysis were prepared by the food codex regulations [13].
Palynological analyses of the samples
The melissopalynological analysis was performed to determine the botanical characteristics of the samples [14]. Botanical characteristics were determined from pollen morphology using light microscopy. The pollen counts were made by adding Lycopodium spores. It is classified according to the percent of unifloral pollen found in honey: the dominant pollen percentage is greater than 45%, it is defined as monofloral honey, secondary pollen (16–45%), minor important pollen (3–15%), and trace pollen (< 3%) [15].
Physicochemical analyses
The values of pH, moisture, and electrical conductivity (EC) values of the samples were determined according to the IHC harmonized method [16]. The specific optical rotation was determined using a polarimeter (Kruss Optical Activity, Germany) using Carrez reagents (I and II) [17]. The turbidity was measured by (WTW-Turb 550). The device is calibrated by using formazan standard calibration suspensions (1000, 10, 0.002 NTU) whose turbidity is placed equally in the range of interest and read from the scale of the device (SM 2130B, 2017). Turbidity values of honey diluted 1:2 with pure water were read on the calibrated device. The appearance colors of the honey samples were measured by Hunter Lab with tritium (Konica, Minolta, and CM-5, Minolta, Osaka, Japan). L for dark/light (0 black, 100 white), a (− a greenness, + a redness) and b (− blueness, + b yellowness).
Proline values were determined by HPLC–UV (according to the reaction based on the formation of a colored complex between amino acids and ninhydrin [18]. The results were expressed as mg/kg. The sugar compositions of honey were analyzed by HPLC-RID using 10 different sugar standards. 75 mL of ultrapure water was added to 1.0 g of honey sample, after vertexing for 5 min, the volume was made up to 100 ml with acetonitrile. After homogenization, it was filtered with a 0.45 µm filter. It was injected into HPLC-RID, which was calibrated with sugar standards [13].
Invertase activity was measured by spectrophotometric method, based on the hydrolysis of p-nitrophenyl-α-D-glucopyranoside (p-NPG) substrate into glucose and p-nitrophenol in the presence of invertase enzyme [19]. Enzyme activity results were calculated in (U/kg) and the detailed method is described in Şahin et al. [7].
Determination of total phenolic content (TPC)
The Folin Ciocalteu reagent, reduced by the phenolic compounds present in the solution, forms a colored complex [20]. The resulting purple-violet complex creates maximum absorbance at 765 nm. In the measurement made according to the gallic acid standard, the results were given in mg GAE/100 g honey [21].
Determination of condensed tannin (CT)
The principle of the method is based on the formation of red color by tannins with strong acids [22]. Different concentrations of catechin were taken as standard (from 0,03 to 1,0 mg/ml), vanillin was added to 37% HCl, incubated at room temperature for 20 min, and absorbance values were read at 500 nm. The results are given as catechin equivalents (mg CAE/g sample).
Determination of total antioxidant capacity (FRAP)
The total antioxidant capacity test was tested according to the FRAP method. This method is based on the principle that the antioxidant substances in honey reduce the Fe (III)-TPTZ complex to the Fe (II)-TPTZ complex. The standard curve was generated using FeSO4.7H2O in a range of concentrations (from 1000 to 31.25 µmol/mL). The details of the method used in the measurement is given [21].
Statistical analysis
Physicochemical parameters, sugar compositions, phenolic contents, and antioxidant capacities of honey types were expressed as mean and standard deviation and evaluated with three replicates at a 5% error level using One-Way Analysis of Variance (One-way ANOVA). Duncan's multiple comparison test was applied to find out which groups caused the differences between honey types.
In this research, discriminant analysis was conducted in conjunction with PCA analysis to identify key variables with the highest discriminatory capability in categorizing honey types. Discriminant analysis proves valuable in revealing distinctions among diverse classes and establishing connections between variables. The statistical analyses were executed utilizing MINITAB 17.0 software.
Results and discussion
Pollen analyses
Table 2 gives melissopalynological values showing the authentic structures of honey. It can be understood from the botanical differences that the honey belongs to the flora of the four different regions studied. It was determined that high plateau honey, which is the honey of the Kars region, has heterofloral properties. It was determined that the pollen profile detected in Kars honey was compatible with studies in the literature [23]. As a result of the field analysis of chestnut honey, which mostly belongs to the Black Sea region, it was determined that the dominant pollen type was chestnut pollen. It was determined that the honey containing an average of 80% chestnut pollen was monofloral chestnut honey. According to honey codes, for a honey to be monofloral chestnut honey, the dominant pollen rate must be greater than 70%. However, this value is 45% in many blossom honeys [11]. In the study, pollen analyses of honeydew honey from two different regions also showed diversity. Ericacea, Tilia, Plantaginaceae, Fabaceae, Lamiaceae, Asteraceae, and Cistaceae were detected as secondary and minor pollen types in the pine honey samples. While Quercus spp. was determined as major pollen in Kırklareli region honey, Fabaceae, Rhamnaceae, Apiaceae, Ericaceae, and Rosaceae were determined as secondary and minor pollen. The pollen profile of both honeydew honeys was found to be different.
Physicochemical properties
The physicochemical parameters of the four distinct honey groups are outlined in Table 3, presenting values as both minimum and maximum ranges alongside the mean (\(\overline{x }\)±sd). Remarkably, no discernible differences were observed across the various honeys concerning pH and moisture content. The pH values ranged between 3.30 and 5.26, while humidity levels fluctuated between 13.70% and 20%. Furthermore, it was noted that these pH and moisture values were consistent with established honey standards [16]. Significant discrepancies were identified in the electrical conductivity (EC) values among the four distinct classes of honey. Heterofloral blossom honey exhibited the lowest EC, averaging 0.36 mS/cm, within a range of 0.25–0.66 mS/cm. The chestnut honey samples exhibited the highest EC value, averaging at 1.43 ± 0.33 mS/cm. Despite chestnut honey being classified as a blossom honey, its physicochemical properties notably differ from other blossom honeys. Interestingly, while honeydew honeys generally displayed EC values exceeding 1.0 mS/cm, no significant variance was detected between the pine and oak honey samples [8, 24]. Chestnut honey, being categorized as forest honey, often possesses elevated levels of pollens and minerals, potentially contributing to its increased EC value [10, 24]. As per the international honey codex, chestnut honey is expected to exhibit a minimum conductivity value of 0.8 mS/cm [11, 18]. The electrical conductivity (EC) of honey is intricately linked to its mineral density and organic acid content, both of which are higher in chestnut honey. This variant tends to contain elevated levels of minerals such as potassium (K), sodium (Na), magnesium (Mg), calcium (Ca), manganese (Mn), zinc (Zn), and iron (Fe) [25]. The higher EC values observed in secretion honeys such as chestnut honey are often attributed to their origin in forest environments [1, 3].
Specific optical rotation (SOR) is a significant physical parameter in honey, though it's not currently included in honey codex regulations. This property is believed to correlate with the sugar composition present in honey [27, 28]. In our study, we observed positive SOR values in pine and oak honey samples, while blossom honey samples exhibited negative values. Previous research on individual sugar monomers reported a specific rotation value of -92.40 for fructose and + 52.70 for glucose [28]. Moreover, it was noted that nearly all honey monosaccharides, except leucrose, demonstrated positive SOR values [28]. Typically, fructose and glucose constitute the primary sugar types in most honey varieties. Despite the similarity in sugar profiles between pine and blossom honey, the notable difference in SOR values remains an intriguing feature. Understanding the sugar composition of honeydew honey remains a challenge, which complicates linking this characteristic to its sugar content.
Turbidity, defined as the cloudiness or haziness in a liquid due to suspended particles, was explored as a novel physical attribute in this study. Pine honey samples, classified as honeydew honeys, exhibited the highest turbidity values among the samples. Conversely, heterofloral flower honeys were identified as having the highest clarity, contrasting with the turbid nature of pine honey. An intriguing finding emerged as chestnut honey showcased greater turbidity compared to oak honey. This observation was unexpected, considering pine honey's anticipated cloudiness, but aligns with the measurements indicating its higher turbidity than oak honey. Turbidity serves as a significant visual property in honey, offering sensory cues distinguishing between varieties such as pine and chestnut honey. The turbidity observed in honey can stem from various factors. In raw honey, insufficient filtration, elevated pollen content, and limited solubility of oligosaccharides in water are plausible reasons. Additionally, the presence of sugar crystals, proteins, wax, and colloidal substances could contribute to its turbid appearance. The higher turbidity in chestnut honey suggests a potential mixture with honeydew honey, possibly sourced from secretions related to chestnut trees in forested regions. Notably, chestnut and pine honeys, known for their low crystallization tendencies, may exhibit cloudiness due to distinct oligosaccharides and significant pollen content [29,30,31]. The global repertoire of honeydew honeys is notably less diverse compared to blossom honeys. A few forest honeys such as pine, oak, cedar, and fir are secretion honey, and it is not easy to distinguish these honeys among themselves. Differentiating between blossom and honeydew honey often relies on the optical rotation value, recognized as a pivotal parameter. While the optical rotation value can ascertain the classification of honey as honeydew honey, it lacks specificity in identifying the exact type of honeydew honey. Consequently, there is a pressing need for novel parameters to discern and categorize the secretion honey accurately. Turbidity may be an important distinguishing feature in distinguishing both pine and oak honeys with positive optical rotation values. The study results showed that pine honey was cloudier than oak honey [5, 27]. However, turbidity in honey is commonly associated with crystal formation, a phenomenon often observed in various types of honey. However, in the context of pine honey, the primary cause of turbidity is not crystallization; rather, it may be attributed to certain polysaccharides present in honey that exhibit limited solubility in water [30].
Color serves as a pivotal sensory-physical parameter for honey. Its measurement, often based on the Pfund color scale or the three-dimensional CIE Lab color model (Hunter Lab), provides insight into honey characteristics [32]. Hunter L value represents the color intensity between black and white, with lower values indicating darker shades. Multifloral blossom honey emerged as the lightest, while oak honey, among the honeydew variety, exhibited a darker hue compared to pine honey. Despite being a blossom honey, chestnut honey possesses a dark color, attributed to its rich content of phenolic substances [15, 24]. Studies indicate a correlation between mineral density and honey color [33]. Hunter a value signifies the redness or greenness of honey, with lower values indicating greenish hues. Analysis showed that blossom honey exhibited more redness compared to honeydew varieties. Studies have suggested a closer association of honeydew honey with green tones [4, 5]. Hunter b value denotes the yellowness or blueness of honey. While differences among honeys regarding Hunter b values weren't distinct, honeydew honey leaned closer to blue, while blossom honey tended toward yellow tones.
The prominence of proline, an amino acid, in honey stands as a significant quality parameter, albeit the specific minimum levels can vary under different national honey regulations [11, 13, 18, 34]. Among the analyzed honey samples, chestnut honey exhibited the highest proline content. The rationale behind proline's prevalence over other amino acids in honey remains elusive. However, considering proline's significance as a major amino acid in collagen protein-a crucial component of connective tissues like skin, bones, and tendons-its abundance might relate to the foraging behavior of honeybees in search of nectar [26, 35, 36]. In particular, higher proline levels of more than 600 mg/kg in chestnut honey may not be due solely to honeybee activity but may also reflect botanical characteristics of the nectar source [5, 24, 37].
The invertase enzyme, responsible for catalyzing the conversion of sucrose into fructose and glucose, holds significance in identifying raw honey, although its activity is not incorporated into honey codex values. This study aimed to ascertain whether invertase activity serves as a distinctive factor between blossom and honeydew honey [7]. The observed invertase activity ranged between 182 and 479 (U/kg), with the highest average value noted in pine honey. However, despite this broad range of activity, it was determined that invertase activity did not serve as a distinguishing parameter between honeydew and blossom honey. These findings suggest that, despite the varying levels of invertase activity, it might not play a differentiating role between these honey types.
The sugar profiles of various honey types, including honeydew and blossom honey, were assessed across nine sugar types. The study revealed a substantial variation in the average fructose content across different honeys, ranging notably from 29 to 41%. The pine honey exhibited the lowest fructose content among the samples. Fructose and glucose stood out as the primary monosaccharides in this honey, while the remaining seven sugar species were detected in trace amounts in some samples and were absent in others [38]. Comparing the honeydew honey to the blossom honey, it was evident that the honeydew honey presented a lower sugar content, which aligns with the established knowledge that honeydew honey typically possesses a lower F + G ratio compared to blossom honey [26]. The F/G ratio, ranging from 1.2 to 1.52, was highest in the pine honey and lowest in the multifloral blossom honey. A lower F/G ratio often correlates with higher honey crystallization rates, potentially influencing the tendency of secretion honey, which doesn't easily crystallize [39]. The chestnut and the honeydew honey, with F/G ratios greater than 1.3, are known for their resistance to crystallization [4, 5, 26]. Moreover, it is asserted that the elevated fructose-to-glucose (F/G) ratio influences the glycemic index of honey, preventing a sudden surge in blood sugar levels. The observation that chestnut, pine, and oak honey exhibit higher F/G ratios compared to multifloral honey suggests that these honeys may be particularly advantageous for individuals with type II diabetes [40,41,42]. While sucrose wasn't detected in some samples and existed in minute amounts in others, in all cases, the sucrose values complied with honey codex standards, registering below 5% [8, 26]. There were no notable differences between honeydew and blossom honey regarding sucrose content. Mannose levels peaked in pine honey at 2.86%, whereas it remained below 1% in other samples. The pine and the chestnut honey exhibited higher maltose levels compared to other varieties. Galactose remained below 3% across all samples, with multifloral blossom honey recording the lowest value. Arabinose was solely detected in the multifloral blossom honey, albeit in minimal amounts, along with some presence in secretion honey and chestnut honey. Trehalose was found in minute quantities only in oak honey, while xylose remained below 0.3% across all samples, with the highest amount observed in the pine honey.
Total phenolic substance contents and antioxidant values are given in Table 4. The average total phenolic content ranged from 30 to 83 mg GAE/100 g across the honey samples, with chestnut honey exhibiting the highest value. The oak honey demonstrated a similar total phenolic content to the chestnut honey, whereas lighter-colored blossom honey showed significantly lower phenolic levels compared to honeydew honey. Notably, chestnut honey, known for its high polyphenol content, signifies substantial potential in apitherapy [37]. Surprisingly, oak honey, akin to chestnut honey, displayed elevated phenolic substance levels. The total tannin content mirrored the trends observed in total phenolic content, with the chestnut and the oak honey boasting higher levels, while the multifloral blossom honey exhibited lower tannin levels. This implies that the major phenolic constituents in the chestnut and the oak honey are condensed tannins. Numerous studies have consistently highlighted a notable correlation between honey color and total phenolic content (TPC), a correlation reaffirmed by the findings in this study [21, 43].
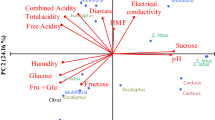
In this study, principal component analysis (PCA) was employed to assess whether phenolic compounds could effectively categorize honey (Fig. 1). The first principal component (PC1) and the second component (PC2) account for 39% and 33% of the overall variance, respectively. Upon examination of the score plot graph, a significant distinction is observed among chestnut (brown), heterofloral (pink), and honeydew (green) honey, with each forming distinct clusters (Fig. 2). Analysis of the loading graph revealed variations in TP, FRAP, SOR, Turbidity, EC, and Hunter L variables between heterofloral blossom and honeydew, excluding chestnut honey. Based on the score plot graphic outcomes, notable distinctions were identified among chestnut honey, heterofloral honey, and pine honey. FRAP, TPC, SOR, Turbidity, and EC values emerged as influential variables in discriminating between the oak and pine honey. Additionally, the clustering of heterofloral blossom honeys was observed to be associated with the Hunter L color value (Fig. 2). Two discriminant functions were identified as applicable for classifying three distinct honey groups. The first discriminant function, featuring a canonical correlation value of 0.93, proved significant as it accounted for approximately 75% of the variances in honey types (Table 5). Canonical discriminant function coefficients for the variables corresponding to functions are also presented in Table 6.
Moreover, the principal component analysis (PCA) is a dimensionality reduction technique used to understand the variability structure in multidimensional data sets and reduce the data size. PCA transforms the variables in the data set into new variables that are not related to each other, and these variables become linear combinations that try to represent the information in the original data set. With this analysis, unnecessary information in the data set (i.e. unnecessary connections between variables) is reduced, the basic structure is preserved, and the basic features of the data set are highlighted [44, 45]. In this study, the PCA analysis was used to classify honey samples according to physicochemical parameters.
In this investigation, the coefficients of the Linear Discriminant Function for Groups were scrutinized to assess the effectiveness of the variables in classifying honey types (Table 6). The table reveals that EC and FRAP variables exhibit the highest coefficients, signifying their substantial discriminatory power in categorizing honey groups. Notably, the Hunter L variable demonstrated a negative impact on Function 1 compared to other variables (Table 6). These findings align with and corroborate the insights derived from the Loading plot in the PCA analysis.
The results of the discriminant analysis yielded accurate results in estimating the group memberships for the selected honey types, as outlined in Table 5. Specifically, one sample from the chestnut honey class and two samples from the oak and pine honey class were misclassified. Conversely, all samples within the heterofloral honey group were correctly assigned to their respective category. The study attains a classification accuracy rate of 96.4% for honey groups, underscoring the high precision in segregating the honey types into three distinct classes in this research.
The correct classification rate obtained because of discriminant analysis determines the success of the analysis. Predicted group membership results for honey types are shown in Table 7. As seen in the classification Table, 1 sample from the chestnut honey group and 2 samples from the oak and pine honey group were grouped incorrectly. All samples in the heterofloral honey group were assigned to the correct group. In the study, the proportion correct for the classification of honey groups was found to be 96.4%. Thus, it can be stated that the accuracy in dividing the honey groups into three classes in this research is high.
In conclusion
In this study, the distinctive features of honeydew and blossom honeys were investigated. Pine and oak honeys were used as honeydew honey, and chestnut and heterofloral flower honeys were used as blossom honeys. It was determined that the specific optical rotation (SOR) value was the most important distinguishing factor among the physicochemical parameters studied between honeydew and blossom honeys. The turbidity value, which reflects the visual value of honey, is an important physical parameter in distinguishing between pine honey and oak honey, which are honeydew honeys. It was determined that the electrical conductivity (EC) value is an important distinguishing factor in honeydew honeys except for chestnut honey. It was determined that low F + G ratios are an important distinguishing feature for honeydew honeys. It was determined that honeydew honeys, except chestnut honey, were darker in color and had more phenolic substances. As a result, the most important outcome of this study was that the most important distinguishing features of pine honey were optical rotation and turbidity.
Data availability
No data were used for the research described in the article.
References
Seraglio SKT, Silva B, Bergamo G, Brugnerotto P, Gonzaga LV, Fett R, Costa CO (2019) An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res Inter 119:44–66
Gounari S, Zotos CE, Dafnis SD, Moschidis G, Papadopoulos GK (2023) On the impact of critical factors to honeydew honey production: the case of Marchalina hellenica and pine honey. J Apicul Res 62(2):383–393
Ertop U, Şevik H, Ertop MH (2023) Mineral composition and heavy metal contents of chestnut honey collected from kastamonu region. J Apither Nat 6(2):73–87
Uçurum HÖ, Tepe Ş, Yeşil E, Güney F, Karakuş S, Kolayli S, Çakici N (2023) Characterization of Turkish pine honey according to their geographical origin based on physicochemical parameters and chemometrics. Euro Food Res Tech 249(5):1317–1327
Kolayli S, Can Z, Çakir HE, Okan OT, Yildiz O (2018) An investigation on Trakya region Oak (Quercus spp) honeys of Turkey: their physico-chemical antioxidant and phenolic compounds properties. Turkish J Biochem 43(4):362–374
Karabagias IK, Badeka AV, Kontakos S, Karabournioti S, Kontominas MG (2014) Botanical discrimination of Greek unifloral honeys with physico-chemical and chemometric analyses. Food Chem 165:181–190
Sahin H, Kolayli S, Beykaya M (2020) Investigation of variations of invertase and glucose oxidase degrees against heating and timing options in raw honeys. J Chem 2020:1–7
Can Z, Yildiz O, Sahin H, Turumtay EA, Silici S, Kolayli S (2015) An investigation of Turkish honeys: their physico-chemical properties antioxidant capacities and phenolic profiles. Food Chem 180:133–141
Sorkun K (2008) Türkiye’nin nektarlı bitkileri polenleri ve balları. Palme Yayıncılık, Ankara
Yılmaz K, Ceylan E, Derelioğlu G (2022) Determination of chestnut honey consumption characteristics as a traditional treatment method and its effect on protection from COVID-19. Comp Med Res 29(3):205–212
Directive C (2001) 110/EC of 20 December 2001 relating to honey. Official J Eur Communities 10(12.1):2002
Güneş ME (2021) Chestnut honey as a complementary medicine: determination of antibacterial activity heavy metal residue and health risk assessment. J Advan Vet Bio Sci Tech 6(2):82–89
Alnuwaiser MA (2017) Content of sugars in fresh grapes and raisins, and fresh and dried apricot: a comparative study. Int J Res Granthaalayah 5:177–186
Louveaux J, Maurizio A, Vorwohl G (1978) Methods of melissopalynology. Bee World 59(4):139–157
Kolayli S, Can Z, Yildiz O, Sahin H, Karaoglu SA (2016) A comparative study of the antihyaluronidase antiurease antioxidant antimicrobial and physicochemical properties of different unifloral degrees of chestnut (Castanea sativa Mill) honeys. J Enzyme Inhib Med Chem 31(sup3):96–104
Bogdanov S, Martin P, Lullmann C (2002) Harmonised methods of the international honey commission. Swiss Bee Res Centre FAM Liebefeld 5:1–62
Bogdanov S, Martin P, Lullmann C, Borneck R, Flamini C, Morlot M, Lheritier J, Vorwohl G, Russmann H, Persano L, Sabatini AG, Marcazzan G, Marioleas P, Tsigouri A, Kerkvliet J, Ortiz A, Ivanov T (1997) Harmonised methods of the European Honey Commission. Apidologie (extra issue):1–59
Sudzina M, Melich M, Kňazovická V, Felšöciov, S, Kociubinski GL, Andreji J, Kráčmar S (2009) Physicochemical characterization of natural honeys from different regions in Slovakia. Acta Univer Agricul Silvicult Mendelianae Brunensis 57.
Oddo LP, Piazza MG, Pulcini P (1999) Invertase activity in honey. Apidologie 30(1):57–65
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent Methods in enzymology. Academic press, Cambridge, pp 152–178
Uçar M, Kemal M, Kanbur ED, Kara Y, Özcelik AE, Kolaylı S (2023) The botanical physicochemical and biochemical characteristics of Northern Cyprus honeys. Euro Food ResTechnol 249(6):1531–1541
Julkunen-Tiitto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33(2):213–217
Ozenirler C, Bayram NE, Celemli OG, Celikbicak O, Sorkun K (2018) Chemical characterization of Kars honey. Fresenius Environ Bull 27(3):1889–1895
Saral Ö (2023) An investigation into chestnut honeys from Artvin province in Turkiye: their physicochemical properties phenolic profiles and antioxidant activities. Chem Biodiver 20(3):e202201162
Kanbur ED, Yuksek T, Atamov V, Ozcelik AE (2021) A comparison of the physicochemical properties of chestnut and highland honey: the case of Senoz Valley in the Rize province of Turkey. Food Chem 345:128864
Manzanares AB, García ZH, Galdón BR, Rodríguez ER, Romero CD (2011) Differentiation of blossom and honeydew honeys using multivariate analysis on the physicochemical parameters and sugar composition. Food Chem 126(2):664–672
Gerginova D, Kurteva V, Simova S (2022) Optical rotation—a reliable parameter for authentication of honey? Molecules 27(24):8916
Serrano S, Rodríguez I, Moreno R, Rincón F (2019) Detection of key factors affecting specific optical rotation determination in honey. CyTA-J Food 17(1):574–580
Basavarajappa S, Raghunandan KS, Hegde SN (2011) Physico-biochemical analysis of multifloral honey of Apis dorsata Fab (hymenoptera: apidae) in southern Karnataka India. Current Biotica 5(2):144–156
Fuad AMA, Anwar NZR, Zakaria AJ, Shahidan N, Zakaria Z (2017) Physicochemical characteristics of Malaysian honeys influenced by storage time and temperature. J Fundam App Sci 9(2S):841–851
Escuredo O, Dobre I, Fernández-González M, Seijo MC (2014) Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem 149:84–90
Al-Farsi M, Al-Amri A, Al-Hadhrami A, Al-Belushi S (2018) Color flavonoids phenolics and antioxidants of Omani honey. Heliyon. https://doi.org/10.1016/j.heliyon.2018.e00874
González-Miret ML, Terrab A, Hernanz D, Fernández-Recamales MÁ, Heredia FJ (2005) Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J Agric Food Chem 53(7):2574–2580
Saroğlu Ö, EcemBayram N, Özçelik B (2023) Comparison of bioactive constituents by HPLC–DAD–ESI-MS and UFLC and in vitro antioxidant activities of blossom honeybee pollen and propolis. Euro Food Res Technol 249(12):3085–3096
Micheu S, Crailsheim K, Leonhard B (2000) Importance of proline and other amino acids during honeybee flight: (Apis mellifera carnica POLLMANN). Amino Acids 18:157–175
Stec N, Saleem A, Darveau CA (2021) Proline as a sparker metabolite of oxidative metabolism during the flight of the bumblebee bombus impatiens. Metabolites 11(8):511
Božič J, Bertoncelj J, Drobne D, Glavan G, Cimerman NG, Leonardi A, Zabret A (2020) The quality of slovenian chestnut honey and its specific properties relevant for medical application and functional nutrition. Acta Biol Sloven 63(2):31–44
Tedesco R, Barbaro E, Zangrando R, Rizzoli A, Malagnini V, Gambaro A, Capodaglio G (2020) Carbohydrate determination in honey samples by ion chromatography–mass spectrometry (HPAEC-MS). Anal Bioanal Chem 412:5217–5227
Buba F, Gidado A, Shugaba A (2013) Analysis of biochemical composition of honey samples from north-east nigeria biochem. Anal Biochem 2(3):139
Zamanian M, Azizi-Soleiman F (2020) Honey and glycemic control: a systematic review. Pharma Nutr 11:100180
Gourdomichali T, Papakonstantinou E (2018) Short-term effects of six Greek honey varieties on glycemic response: a randomized clinical trial in healthy subjects. Euro J Clin Nut 72(12):1709–1716
Bobiş O, Dezmirean DS, Moise AR (2018) Honey and diabetes: the importance of natural simple sugars in diet for preventing and treating different type of diabetes. Oxid Med Cell Longev. https://doi.org/10.1155/2018/4757893
Kemal M, Üreyen E, Kanbur ED, Kara Y, Özçelik AE, Can Z, Kolaylı S (2023) Characterization of the black cumin (Nigella sativa L) honey from Türkiye. Food Biosci 53:102760
Jolliffe IT (1986) Principal component analysis and factor analysis in principal component analysis. Springer, New York, pp 115–128
Shlens J (2014) A tutorial on principal component analysis. Ar**v. https://doi.org/10.48550/ar**v.1404.1100
Acknowledgements
We extend our gratitude to the TAGEM project office of the Ministry of Food, Agriculture, and Forestry for their invaluable support of our projects. (TAGEM/HSGYAD/Ü/20/A3/P1/1464).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
OU: Preparation and execution of the project HK: palynological analysis, PMB: sugar analyses, MB: total fiber analyses, CT: all statistics, SK: conceptualization, methodology, writing.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest between the authors.
Ethical approval
It is not a study that requires an ethics committee decision.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ucurum, O., Tosunoglu, H., Takma, Ç. et al. Distinctive properties of the pine, oak, chestnut and multifloral blossom and honeydew honeys. Eur Food Res Technol 250, 1765–1774 (2024). https://doi.org/10.1007/s00217-024-04520-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-024-04520-0