Abstract
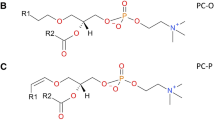
Plasmalogens are a special class of glycerophospholipids characterized by a vinyl ether bond (-C = C-O-) at the sn-1 position of the glycerol backbone. Altered plasmalogen profiles have been observed in neurodegenerative diseases and cancers. Profiling of plasmalogens requires specifying the vinyl ether bond and differentiating them from various types of isobars and isomers. Herein, by coupling C = C derivatization via offline Paternò–Büchi reaction with liquid chromatography-tandem mass spectrometry, we have developed a sensitive workflow for analysis of plasmalogens from biological samples. Using bovine heart lipid extract as a model system, we profiled more than 100 distinct structures of plasmenylethanolamines (PE-Ps) and plasmenylcholines (PC-Ps) at the C = C location level, far exceeding previous reports. Analysis of human glioma and normal brain tissue samples revealed elevated n-10 C = C isomers of PE-Ps in the glioma tissue samples. These findings suggest that the developed workflow holds potential in aiding the study of altered metabolism of plasmalogens in clinical samples.
Graphical Abstract







Similar content being viewed by others
References
Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822(9):1442–52.
Astudillo AM, Balboa MA, Balsinde J. Compartmentalized regulation of lipid signaling in oxidative stress and inflammation: plasmalogens, oxidized lipids and ferroptosis as new paradigms of bioactive lipid research. Prog Lipid Res. 2023;89:101207.
Stradomska TJ, Syczewska M, Jamroz E, Pleskaczynska A, Kruczek P, Ciara E, et al. Serum very long-chain fatty acids (VLCFA) levels as predictive biomarkers of diseases severity and probability of survival in peroxisomal disorders. PLoS ONE. 2020;15(9):e0238796.
Steinberg SJ, Dodt G, Raymond GV, Braverman NE, Moser AB, Moser HW. Peroxisome biogenesis disorders. Biochim Biophys Acta. 2006;1763(12):1733–48.
Han X, Holtzman DM, McKeel DW Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem. 2001;77(4):1168–80.
Wood PL, Locke VA, Herling P, Passaro A, Vigna GB, Volpato S, et al. Targeted lipidomics distinguishes patient subgroups in mild cognitive impairment (MCI) and late onset Alzheimer’s disease (LOAD). BBA Clin. 2016;5:25–8.
Patterson NH, Alabdulkarim B, Lazaris A, Thomas A, Marcinkiewicz MM, Gao ZH, et al. Assessment of pathological response to therapy using lipid mass spectrometry imaging. Sci Rep. 2016;6:36814.
Benjamin DI, Cozzo A, Ji X, Roberts LS, Louie SM, Mulvihill MM, et al. Ether lipid generating enzyme AGPS alters the balance of structural and signaling lipids to fuel cancer pathogenicity. Proc Natl Acad Sci U S A. 2013;110(37):14912–7.
Lv J, Lv CQ, Xu L, Yang H. Plasma content variation and correlation of plasmalogen and GIS, TC, and TPL in gastric carcinoma patients: a comparative study. Med Sci Monit Basic Res. 2015;21:157–60.
Rustam YH, Reid GE. Analytical challenges and recent advances in mass spectrometry based lipidomics. Anal Chem. 2018;90(1):374–97.
Holcapek M, Liebisch G, Ekroos K. Lipidomic analysis. Anal Chem. 2018;90(7):4249–57.
Ryan E, Reid GE. Chemical derivatization and ultrahigh resolution and accurate mass spectrometry strategies for “shotgun” lipidome analysis. Accounts Chem Res. 2016;49(9):1596–604.
Zemski Berry KA, Murphy RC. Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J Am Soc Mass Spectr. 2004;15(10):1499–508.
Blevins MS, Shields SWJ, Cui W, Fallatah W, Moser AB, Braverman NE, et al. Structural characterization and quantitation of ether-Linked glycerophospholipids in peroxisome biogenesis disorder tissue by ultraviolet photodissociation mass spectrometry. Anal Chem. 2022;94(37):12621–9.
Hsu FF, Lodhi IJ, Turk J, Semenkovich CF. Structural distinction of diacyl-, alkylacyl, and alk-1-enylacyl glycerophosphocholines as [M - 15](-) ions by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectr. 2014;25(8):1412–20.
Fhaner CJ, Liu S, Zhou X, Reid GE. Functional group selective derivatization and gas-phase fragmentation reactions of plasmalogen glycerophospholipids. Mass Spectrom (Tokyo). 2013;2(Spec Iss):S0015.
Lydic TA, Townsend S, Adda CG, Collins C, Mathivanan S, Reid GE. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods. 2015;87:83–95.
Zhang W, Jian R, Zhao J, Liu Y, ** by mass spectrometry: recent technical advances and applications. J Lipid Res. 2022;63(7): 100219.
Deeley JM, Thomas MC, Truscott RJW, Mitchell TW, Blanksby SJ. Identification of abundant alkyl ether glycerophospholipids in the human lens by tandem mass spectrometry techniques. Anal Chem. 2009;81(5):1920–30.
Marshall DL, Criscuolo A, Young RSE, Poad BLJ, Zeller M, Reid GE, et al. Map** unsaturation in human plasma lipids by data-independent ozone-induced dissociation. J Am Soc Mass Spectr. 2019;30(9):1621–30.
Baba T, Campbell JL, Le Blanc JCY, Baker PRS, Ikeda K. Quantitative structural multiclass lipidomics using differential mobility: electron impact excitation of ions from organics (EIEIO) mass spectrometry. J Lipid Res. 2018;59(5):910–9.
Lin Q, Li P, Fang M, Zhang D, **a Y. Deep profiling of aminophospholipids reveals a dysregulated desaturation pattern in breast cancer cell lines. Anal Chem. 2022;94(2):820–8.
Lin Q, Zhang D, **a Y. Analysis of ether glycerophosphocholines at the level of C=C locations from human plasma. Analyst. 2020;145(2):513–22.
Liebisch G, Fahy E, Aoki J, Dennis EA, Durand T, Ejsing CS, et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J Lipid Res. 2020;61(12):1539–55.
Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509.
Zhao J, **e X, Lin Q, Ma X, Su P, **a Y. Next-generation Paternò-Büchi reagents for lipid analysis by mass spectrometry. Anal Chem. 2020;92(19):13470–7.
Shi H, Tan Z, Guo X, Ren H, Wang S, **a Y. Visible-light Paternò-Büchi reaction for lipidomic profiling at detailed structure levels. Anal Chem. 2023;95(11):5117–25.
Cao W, Cheng S, Yang J, Feng J, Zhang W, Li Z, et al. Large-scale lipid analysis with C=C location and sn-position isomer resolving power. Nat Commun. 2020;11(1):375.
**e X, Zhao J, Lin M, Zhang JL, **a Y. Profiling of cholesteryl esters by coupling charge-tagging Paternò-Büchi reaction and liquid chromatography-mass spectrometry. Anal Chem. 2020;92(12):8487–96.
Chen Y, **e C, Wang X, Cao G, Ru Y, Song Y, et al. 3-Acetylpyridine on-tissue Paternò-Büchi derivatization enabling high coverage lipid C=C location-resolved MS imaging in biological tissues. Anal Chem. 2022;94(44):15367–76.
Li H-F, Zhao J, Cao W, Zhang W, **a Y, Ouyang Z. Site-specific photochemical reaction for improved C=C location analysis of unsaturated lipids by ultraviolet photodissociation. Research. 2022;2022:9783602.
**a T, Zhou F, Zhang D, ** X, Shi H, Yin H, et al. Deep-profiling of phospholipidome via rapid orthogonal separations and isomer-resolved mass spectrometry. Nat Commun. 2023;14:4263.
Heymans HSA, Schutgens RBH, Tan R, Hvd Bosch, Borst P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature. 1983;306:69–70.
Yang K, Jenkins CM, Dilthey B, Gross RW. Multidimensional mass spectrometry-based shotgun lipidomics analysis of vinyl ether diglycerides. Anal Bioanal Chem. 2015;407(17):5199–210.
Panganamala RV, Horrocks LA, Geer JC, Cornwell DG. Positions of double bonds in the monounsaturated alk-1-enyl groups from the plasmalogens of human heart and brain. Chem Phys Lipids. 1971;6(2):97–102.
Matsushita Y, Nakagawa H, Koike K. Lipid metabolism in oncology: why it matters, how to research, and how to treat. Cancers (Basel). 2021;13(3):474.
Zhu Y, Liu XJ, Yang P, Zhao M, Lv LX, Zhang GD, et al. Alkylglyceronephosphate synthase (AGPS) alters lipid signaling pathways and supports chemotherapy resistance of glioma and hepatic carcinoma cell lines. Asian Pac J Cancer Prev. 2014;15(7):3219–26.
Maimo-Barcelo A, Martin-Saiz L, Fernandez JA, Perez-Romero K, Garfias-Arjona S, Lara-Almunia M, et al. Polyunsaturated fatty acid-enriched lipid fingerprint of glioblastoma proliferative regions is differentially regulated according to glioblastoma molecular subtype. Int J Mol Sci. 2022;23(6):2949.
Funding
Financial support from the National Natural Science Foundation of China (Grant No. 22225404) and National Key R&D Program of China (2018YFA0800903) is greatly appreciated.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.X.; sample preparation: Y.W.; analysis: Y.W.; resources: Y.X.; writing—original draft preparation: Y.W.; writing—review and editing: Y.X.; visualization: Y.W.; supervision: Y.X. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical disclosure and source of biological material
Human brain tissue samples were obtained from Huashan Hospital, affiliated with Fudan University. All protocols involving these biological samples received approval from the Ethical Review Board at Tsinghua University, under the approval number IRB No. 20180030.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection New Trends in Lipidomics with guest editor Michal Holčapek.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., **a, Y. Deep profiling of plasmalogens by coupling the Paternò–Büchi derivatization with tandem mass spectrometry. Anal Bioanal Chem (2024). https://doi.org/10.1007/s00216-024-05376-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00216-024-05376-9