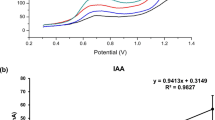
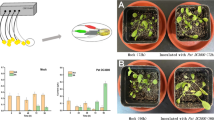
Abstract
Because of the pivotal point of Marchantia polymorpha (M. polymorpha) in plant evolution, its auxin (mainly indole-3-acetic acid, IAA) levels could provide useful evidence for the study of the evolution of IAA. However, M. polymorpha could not be easily pretreated for electrochemical detection because they are at the entry level of land plants. Herein, we designed a three-dimensional (3D)-printed analytical device for seamless integration of sample treatment and electrochemical detection. Specifically, the electrochemical cell could be used as a mortar in which a tiny plant sample could be ground with a 3D-printed pestle, followed by mixing with the buffer solution under vibration for electrochemical detection of IAA with a disposable working electrode at the bottom of the cell. Using our strategy, the limits of quantification could reach 0.05 μmol L−1 after optimization of parameters. We were able to demonstrate that IAA in different tissues of wild-type and mutant M. polymorpha could be successfully differentiated after they were treated with the 3D-printed analytical device. The obtained results were comparable to the samples blended with zirconium beads while the differences of IAA levels in different tissues of M. polymorpha agreed well with previous reports. This study suggested the potential of sample treatment integrated with electrochemical detection for analysis of IAA using the 3D printing techniques and their possible applications in the research of plants and other fields.








Similar content being viewed by others
References
Castro A, Vidal S, de Leon IP (2016) Moss Pathogenesis-Related-10 Protein Enhances Resistance to Pythium irregulare in Physcomitrella patens and Arabidopsis thaliana. Front Plant Sci7. https://doi.org/10.3389/fpls.2016.00580.
Gramzow L, Weilandt L, Theissen G. MADS goes genomic in conifers: towards determining the ancestral set of MADS-box genes in seed plants. Ann Bot. 2014;114(7):1407–29. https://doi.org/10.1093/aob/mcu066.
Imaizumi T, Kadota A, Hasebe M, Wada M. Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens. Plant Cell. 2002;14(2):373–86. https://doi.org/10.1105/tpc.010388.
Paponov IA, Teale W, Lang D, Paponov M, Reski R, Rensing SA, Palme K (2009) The evolution of nuclear auxin signalling. Bmc Evol Biol 9. https://doi.org/10.1186/1471-2148-9-126.
Cooke TJ, Poli D, Sztein AE, Cohen JD. Evolutionary patterns in auxin action. Plant Mol Biol. 2002;49(3–4):319–38. https://doi.org/10.1023/a:1015242627321.
Shimamura M. Marchantia polymorpha: Taxonomy, Phylogeny and Morphology of a Model System. Plant and Cell Physiology. 2015;57:pcv192. https://doi.org/10.1093/pcp/pcv192.
Chandler JW. Local auxin production: a small contribution to a big field. BioEssays. 2009;31(1):60–70. https://doi.org/10.1002/bies.080146.
Cai B-D, Ye E-C, Yuan B-F, Feng Y-Q. Sequential solvent induced phase transition extraction for profiling of endogenous phytohormones in plants by liquid chromatography-mass spectrometry. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2015;1004:23–9. https://doi.org/10.1016/j.jchromb.2015.09.031.
**ao H-M, Cai W-J, Ye T-T, Ding J, Feng Y-Q. Spatio-temporal profiling of abscisic acid, indoleacetic acid and jasmonic acid in single rice seed during seed germination. Anal Chim Acta. 2018;1031:119–27. https://doi.org/10.1016/j.aca.2018.05.055.
Brunoud G, Wells DM, Oliva M, Larrieu A, Mirabet V, Burrow AH, Beeckman T, Kepinski S, Traas J, Bennett MJ, Vernoux T. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature. 2012;482(7383):103-U132. https://doi.org/10.1038/nature10791.
Cao X, Zhu X, He S, Xu X, Ye Y (2019) Electro-Oxidation and Simultaneous Determination of Indole-3-Acetic Acid and Salicylic Acid on Graphene Hydrogel Modified Electrode. Sensors 19 (24). https://doi.org/10.3390/s19245483.
Huo X-L, Qi J-F, He K-C, Bao N, Shi C-G. Stainless steel sheets as the substrate of disposable electrochemical sensors for analysis of heavy metals or biomolecules. Anal Chim Acta. 2020;1124:32–9. https://doi.org/10.1016/j.aca.2020.05.018.
Zhang X, Zhou J, Li Z, Qin Y, Yu R, Zhang H, Zheng Y, Zhu J, Zhang D, Fu L. The Qualitative Electrochemical Determination of Multiple Components in Seaweed Fertilizer. Int J Electrochem Sci. 2019;14(7):6283–91. https://doi.org/10.20964/2019.07.16.
Wu BF, Xu HT, Shi YF, Zhou H, Li YP, Deng HD, Ye JS, Long YB, Lan YB Online Monitoring of Indole-3-acetic Acid in Living Plants Based on Nitrogen-Doped Carbon Nanotubes/Core-shell Au@Cu2O Nanoparticles/Carbon Fiber Electrochemical Microsensor. Acs Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.2c04222.
Rabie EM, Shamroukh AA, Khodari M. A Novel Electrochemical Sensor Based on Modified Carbon Paste Electrode with ZnO Nanorods for the Voltammetric Determination of Indole-3-acetic Acid in Plant Seed Extracts. Electroanalysis. 2022;34(5):883–91. https://doi.org/10.1002/elan.202100420.
Sun L, Xu SZ, Tang YH, Zhou YH, Wang M, Tian YR, Li GX, Zhu XY, Bao N, Sun LJ Disposable stainless steel working electrodes for sensitive and simultaneous detection of indole-3-acetic acid and salicylic acid in Arabidopsis thaliana leaves under biotic stresses. Anal Bioanal Chem. https://doi.org/10.1007/s00216-022-04303-0.
Huo XL, Zhu CC, Jiang H, Yuan Q, Wang JJ, Wang JY, Pan ZQ, Chen CL, Wu ZQ, Bao N (2021) Rapid profiling of IAA and SA in tomato fruit during ripening using low-cost paper-based electroanalytical devices. Postharvest Biol Technol. 180. https://doi.org/10.1016/j.postharvbio.2021.111635.
Li HY, Wang C, Wang XD, Hou PC, Luo B, Song P, Pan DY, Li AX, Chen LP. Disposable stainless steel-based electrochemical microsensor for in vivo determination of indole-3-acetic acid in soybean seedlings. Biosens Bioelectron. 2019;126:193–9. https://doi.org/10.1016/j.bios.2018.10.041.
Cardoso RM, Kalinke C, Rocha RG, dos Santos PL, Rocha DP, Oliveira PR, Janegitz BC, Bonacin JA, Richter EM, Munoz RAA. Additive-manufactured (3D-printed) electrochemical sensors: A critical review. Anal Chim Acta. 2020;1118:73–91. https://doi.org/10.1016/j.aca.2020.03.028.
Hamzah HH, Shafiee SA, Abdalla A, Patel BA. 3D printable conductive materials for the fabrication of electrochemical sensors: A mini review. Electrochem Commun. 2018;96:27–31. https://doi.org/10.1016/j.elecom.2018.09.006.
Sun Q, Wang J, Tang M, Huang L, Zhang Z, Liu C, Lu X, Hunter KW, Chen G. A New Electrochemical System Based on a Flow-Field Shaped Solid Electrode and 3D-Printed Thin-Layer Flow Cell: Detection of Pb2+ Ions by Continuous Flow Accumulation Square-Wave Anodic Strip** Voltammetry. Anal Chem. 2017;89(9):5024–9. https://doi.org/10.1021/acs.analchem.7b00383.
Banna M, Bera K, Sochol R, Lin L, Najjaran H, Sadiq R, Hoorfar M (2017) 3D Printing-Based Integrated Water Quality Sensing System. Sensors 17 (6). https://doi.org/10.3390/s17061336.
Qi L, Yuan F, Wu F, Ma X, Amatore C, Xu G. 3D Printed Rotating Acentric Binary-Disk Electrode. Anal Chem. 2018;90(22):13217–21. https://doi.org/10.1021/acs.analchem.8b03393.
Cardoso RM, Mendonca DMH, Silva WP, Silva MNT, Nossol E, da Silva RAB, Richter EM, Munoz RAA. 3D printing for electroanalysis: From multiuse electrochemical cells to sensors. Anal Chim Acta. 2018;1033:49–57. https://doi.org/10.1016/j.aca.2018.06.021.
Honeychurch KC, Rymansaib Z, Iravani P. Anodic strip** voltammetric determination of zinc at a 3-D printed carbon nanofiber-graphite-polystyrene electrode using a carbon pseudo-reference electrode. Sensors and Actuators B-Chemical. 2018;267:476–82. https://doi.org/10.1016/j.snb.2018.04.054.
Katseli V, Thomaidis N, Economou A, Kokkinos C (2020) Miniature 3D-printed integrated electrochemical cell for trace voltammetric Hg(II) determination. Sens Actuators B-Chem 308. https://doi.org/10.1016/j.snb.2020.127715.
Shergill RS, Farlow A, Perez F, Patel BA (2022) 3D-printed electrochemical pestle and mortar for identification of falsified pharmaceutical tablets. Microchimica Acta. 189 (3). https://doi.org/10.1007/s00604-022-05202-y.
Kitte SA, Li S, Nsabimana A, Gao W, Lai J, Liu Z, Xu G. Stainless steel electrode for simultaneous strip** analysis of Cd(II), Pb(II), Cu(II) and Hg(II). Talanta. 2019;191:485–90. https://doi.org/10.1016/j.talanta.2018.08.066.
Kitte SA, Gao W, Zholudov YT, Qi L, Nsabimana A, Liu Z, Xu G. Stainless Steel Electrode for Sensitive Luminol Electrochemiluminescent Detection of H2O2, Glucose, and Glucose Oxidase Activity. Anal Chem. 2017;89(18):9864–9. https://doi.org/10.1021/acs.analchem.7b01939.
Heald FDF. Conditions for the Germination of the Spores of Bryophytes and Pteridophytes. Bot Gaz. 1898;26(1):25–45. https://doi.org/10.1086/327712.
Nakazato T, Kadota A, Wada M (1999) Photoinduction of Spore Germination in Marchantia polymorpha L. is Mediated by Photosynthesis. Plant Cell Phys. 40. https://doi.org/10.1093/oxfordjournals.pcp.a029482.
Sun L-J, Zhou J-J, Pan J-L, Liang Y-Y, Fang Z-J, ** of indole-3-acetic acid and salicylic acid in whole pea seedlings under normal conditions and salinity. Sens Actuators, B Chem. 2018;276:545–51. https://doi.org/10.1016/j.snb.2018.08.152.
Sun L-J, **e Y, Yan Y-F, Yang H, Gu H-Y, Bao N. Paper-based analytical devices for direct electrochemical detection of free IAA and SA in plant samples with the weight of several milligrams. Sens Actuators, B Chem. 2017;247:336–42. https://doi.org/10.1016/j.snb.2017.03.025.
Lee D, Yang S. Surface modification of PDMS by atmospheric-pressure plasma-enhanced chemical vapor deposition and analysis of long-lasting surface hydrophilicity. Sensors and Actuators B-Chemical. 2012;162(1):425–34. https://doi.org/10.1016/j.snb.2011.12.017.
Hernandez P, Galan F, Nieto O, Hernandez L. Direct determination of indole-3-acetic acid in plant tissues by electroanalytical techniques using a carbon paste modified with OV-17 electrode. Electroanalysis. 1994;6(7):577–83. https://doi.org/10.1002/elan.1140060708.
Wang H-R, Bi X-M, Fang Z-J, Yang H, Gu H-Y, Sun L-J, Bao N. Real time sensing of salicylic acid in infected tomato leaves using carbon tape electrodes modified with handed pencil trace. Sens Actuators, B Chem. 2019;286:104–10. https://doi.org/10.1016/j.snb.2019.01.119.
Eklund DM, Ishizaki K, Flores-Sandoval E, Kikuchi S, Takebayashi Y, Tsukamoto S, Hirakawa Y, Nonomura M, Kato H, Kouno M, Bhalerao RP, Lagercrantz U, Kasahara H, Kohchi T, Bowman JL. Auxin Produced by the Indole-3-Pyruvic Acid Pathway Regulates Development and Gemmae Dormancy in the Liverwort Marchantia polymorph. Plant Cell. 2015;27(6):1650–69. https://doi.org/10.1105/tpc.15.00065.
Funding
This work received financial support from the National Natural Science Foundation of China (No.: 32070397 and 21904072).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, XY., Wang, YH., Liu, W. et al. A 3D-printed analytical device seamlessly integrating sample treatment for electrochemical detection of IAA in Marchantia polymorpha. Anal Bioanal Chem 415, 1385–1393 (2023). https://doi.org/10.1007/s00216-023-04529-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04529-6