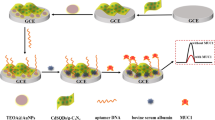
Abstract
A mixed aptamer-antibody sandwich assay for the determination of mucin protein 16 (MUC16) was developed based on hybridization chain reaction (HCR) with methylene blue (MB) as an electrochemical indicator. First, MUC16 antibody was adsorbed onto the surface of the Au nanoparticle (AuNP)-modified indium tin oxide (ITO) electrode to effectively capture the target MUC16. After MUC16 was captured by the MUC16 aptamer, an antibody/MUC16/aptamer sandwich structure formed for the highly selective detection of MUC16. The 3′ end of the aptamer was then subjected to HCR with the assistance of auxiliary probes to obtain DNA concatemers. Numerous MB molecules bonded with G bases in the DNA concatemers by immersing the modified ITO electrode into a stirred solution containing MB with KCl. Stepwise changes in the microscopic features of the electrode surface were studied by scanning electron microscopy (SEM). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were used to characterize the electrochemical behavior of the different modified electrodes. The oxidation current of MB was detected by differential pulse voltammetry (DPV). Under the optimum conditions, the proposed mixed aptamer-antibody sandwich assay showed wide dynamic range from 0.39 to 200 unit mL−1 with a low detection limit of 0.02 unit mL−1 (S/N ratio = 3). The proposed method showed good accuracy, selectivity, and acceptable reproducibility.

Graphical abstract
An electrochemical mixed aptamer-antibody sandwich assay based on the aptamer-induced HCR amplification strategy was fabricated for the highly sensitive detection of MUC16. The mixed aptamer-antibody sandwich assay showed acceptable performance of detection range, detection limit, reproducibility, and selectivity.








Similar content being viewed by others
References
Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, et al. Functions of MUC16 in corneal epithelial cells. Investig Ophthalmol Vis Sci. 2007;48:4509–18.
Perez BH, Gipson IK. Focus on molecules: human mucin MUC16. Exp Eye Res. 2008;87:400–1.
Diaconu I, Cristea C, Hârceagă V, Marrazza G, Berindan-Neagoe I, Săndulescu R. Electrochemical immunosensors in breast and ovarian cancer. Clin Chim Acta. 2013;425:128–38.
Taleat Z, Cristea C, Marrazza G, Mazloum-Ardakani M, Sǎndulescu R. Electrochemical immunoassay based on aptamer–protein interaction and functionalized polymer for cancer biomarker detection. J Electroanal Chem. 2014;717–718:119–24.
Ricardo S, Marcos-Silva L, Pereira D, Pinto R, Almeida R, Söderberg O, et al. Detection of glyco–mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Mol Oncol. 2015;9:503–12.
Gedi V, Song CK, Kim GB, Lee JO, Oh E, Shin BS, et al. Sensitive on–chip detection of cancer antigen 125 using a DNA aptamer/carbon nanotube network platform. Sensors Actuators B Chem. 2018;256:89–97.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–10.
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–22.
Aydoğdu Tığ G, Pekyardımcı Ş. An electrochemical sandwich–type aptasensor for determination of lipocalin–2 based on graphene oxide/polymer composite and gold nanoparticles. Talanta. 2020;210:120666.
Eaton RM, Shallcross JA, Mael LE, Mears KS, Minkoff L, Scoville DJ, et al. Selection of DNA aptamers for ovarian cancer biomarker HE4 using CE–SELEX and high–throughput sequencing. Anal Bioanal Chem. 2015;407:6965–73.
Gong W, Duan N, Wu S, Huang Y, Chen X, Wang Z. Selection, identification, and application of dual DNA aptamers against Shigella sonnei. Anal Methods. 2015;7:3625–31.
Wang J, Wang F, Dong S. Methylene blue as an indicator for sensitive electrochemical detection of adenosine based on aptamer switch. J Electroanal Chem. 2009;626:1–5.
Liu H, Xu S, He Z, Deng A, Zhu JJ. Supersandwich cytosensor for selective and ultrasensitive detection of cancer cells using aptamer–DNA concatemer–quantum dots probes. Anal Chem. 2013;85:3385–92.
Hashkavayi AB, Raoof JB, Ojani R, Kavoosian S. Ultrasensitive electrochemical aptasensor based on sandwich architecture for selective label–free detection of colorectal cancer (CT26) cells. Biosens Bioelectron. 2017;92:630–7.
Hashkavayi AB, Raoof JB. Design an aptasensor based on structure-switching aptamer on dendritic gold nanostructures/Fe3O4@SiO2/DABCO modified screen printed electrode for highly selective detection of epirubicin. Biosens Bioelectron. 2017;91:650–7.
Wang H, Chen H, Huang Z, Li T, Deng A, Kong J. DNase I enzyme–aided fluorescence signal amplification based on graphene oxide–DNA aptamer interactions for colorectal cancer exosome detection. Talanta. 2018;184:219–26.
Park KS. Nucleic acid aptamer–based methods for diagnosis of infections. Biosens Bioelectron. 2018;102:179–88.
Evtugyn G, Hianik T. Electrochemical DNA sensors and aptasensors based on electropolymerized materials and polyelectrolyte complexes. TrAC–Trends Anal Chem. 2016;79:168–78.
Qureshi A, Gurbuz Y, Niazi JH. Capacitive aptamer–antibody based sandwich assay for the detection of VEGF cancer biomarker in serum. Sensors Actuators B Chem. 2015;209:645–51.
Florea A, Taleat Z, Cristea C, Mazloum–Ardakani M, Sǎndulescu R. Label free MUC1 aptasensors based on electrodeposition of gold nanoparticles on screen printed electrodes. Electrochem Commun. 2013;33:127–30.
Wang F, Gopinath SCB, Lakshmipriya T. Aptamer–antibody complementation on multiwalled carbon nanotube–gold transduced dielectrode surfaces to detect pandemic swine influenza virus. Int J Nanomedicine. 2019;14:8469–81.
Yao J, Li S, Zhang L, Yang Y, Gopinath SCB, Lakshmipriya T, et al. Aptamer–antibody dual probes on single–walled carbon nanotube bridged dielectrode: comparative analysis on human blood clotting factor. Int J Biol Macromol. 2020;151:1133–8.
Xu H, Kou F, Ye H, Wang Z, Huang S, Liu X, et al. Highly sensitive antibody–aptamer sensor for vascular endothelial growth factor based on hybridization chain reaction and pH meter/indicator. Talanta. 2017;175:177–82.
Wu W, Li J, Pan D, Li J, Song S, Rong M, et al. Gold nanoparticle–based enzyme–linked antibody–aptamer sandwich assay for detection of salmonella typhimurium. ACS Appl Mater Interfaces. 2014;6:16974–81.
Hosseini Ghalehno M, Mirzaei M, Torkzadeh-Mahani M. Electrochemical aptasensor for tumor necrosis factor α using aptamer–antibody sandwich structure and cobalt hexacyanoferrate for signal amplification. J Iran Chem Soc. 2019;16:1783–91.
Zhou L, Ou LJ, Chu X, Shen GL, Yu RQ. Aptamer–based rolling circle amplification: a platform for electrochemical detection of protein. Anal Chem. 2007;79:7492–500.
Teng J, Ye Y, Yao L, Yan C, Cheng K, Xue F, et al. Rolling circle amplification based amperometric aptamer/immuno hybrid biosensor for ultrasensitive detection of Vibrio parahaemolyticus. Microchim Acta. 2017;184:3477–85.
Niemeyer CM, Adler M, Wacker R. Immuno–PCR: high sensitivity detection of proteins by nucleic acid amplification. Trends Biotechnol. 2005;23:208–16.
Zhu X, Kou F, Xu H, Lin L, Yang G, Lin Z. A highly sensitive aptamer–immunoassay for vascular endothelial growth factor coupled with portable glucose meter and hybridization chain reaction. Sensors Actuators B Chem. 2017;253:660–5.
Sun H, Yao F, Su Z, Kang XF. Hybridization chain reaction (HCR) for amplifying nanopore signals. Biosens Bioelectron. 2020;150:111906.
Jia LP, Zhao RN, Wang LJ, Ma RN, Zhang W, Shang L, et al. Aptamer based electrochemical assay for protein kinase activity by coupling hybridization chain reaction. Biosens Bioelectron. 2018;117:690–5.
Yang W, Ozsoz M, Hibbert DB, Gooding JJ. Evidence for the direct interaction between methylene blue and guanine bases using DNA–modified carbon paste electrodes. Electroanalysis. 2002;14:1299–302.
Wain AJ, Zhou F. Scanning electrochemical microscopy imaging of DNA microarrays using methylene blue as a redox–active intercalator. Langmuir. 2008;24:5155–60.
Rafiee-Pour HA, Behpour M, Keshavarz M. A novel label–free electrochemical miRNA biosensor using methylene blue as redox indicator: application to breast cancer biomarker miRNA–21. Biosens Bioelectron. 2016;77:202–7.
**g X, Cao X, Wang L, Lan T, Li Y, **e G. DNA–AuNPs based signal amplification for highly sensitive detection of DNA methylation, methyltransferase activity and inhibitor screening. Biosens Bioelectron. 2014;58:40–7.
Wang J, Wang L, Di J, Tu Y. Disposable biosensor based on immobilization of glucose oxidase at gold nanoparticles electrodeposited on indium tin oxide electrode. Sensors Actuators B Chem. 2008;135:283–8.
Lu S, Hu T, Wang S, Sun J, Yang X. Ultra–sensitive colorimetric assay system based on the hybridization chain reaction–triggered enzyme cascade amplification. ACS Appl Mater Interfaces. 2017;9:167–75.
Liu P, Yang X, Sun S, Wang Q, Wang K, Huang J, et al. Enzyme–free colorimetric detection of DNA by using gold nanoparticles and hybridization chain reaction amplification. Anal Chem. 2013;85:7689–95.
Chen F, Liu Y, Chen C. Respective and simultaneous detection tumor markers CA125 and STIP1 using aptamer–based fluorescent and RLS sensors. Sensors Actuators B Chem. 2017;245:470–6.
Samadi Pakchin P, Ghanbari H, Saber R, Omidi Y. Electrochemical immunosensor based on chitosan–gold nanoparticle/carbon nanotube as a platform and lactate oxidase as a label for detection of CA125. Biosens Bioelectron. 2018;122:68–74.
Wu L, Sha Y, Li W. One–step preparation of disposable multi–functionalized g–C3N4 based electrochemiluminescence immunosensor for the detection of CA125. Sensors Actuators B Chem. 2016;226:62–8.
Raghav R, Srivastava S. Core–shell gold–silver nanoparticles based impedimetric immunosensor for cancer antigen CA125. Sensors Actuators B Chem. 2015;220:557–64.
Hamd-Ghadareh S, Salimi A, Fathi F, Bahrami S. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio–applications in labeling, imaging and sensing. Biosens Bioelectron. 2017;96:308–16.
** H, Gui R, Gong J, Huang W. Aptamer and 5–fluorouracil dual–loading Ag2S quantum dots used as a sensitive label–free probe for near–infrared photoluminescence turn–on detection of CA125 antigen. Biosens Bioelectron. 2017;92:378–84.
Ravalli A, Pilon Dos Santos G, Ferroni M. New label free CA125 detection based on gold nanostructured screen–printed electrode. Sensors Actuators B Chem. 2013;179:194–200.
Funding
This research was financially supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2017PY024 and No. 2018PY022), the Zhejiang Provincial Natural Science Foundation of China (LQ19H090019), and the Public Welfare from the Science and Technology Department of Zhejiang Province (No. 2017C33201).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, and were approved by the Ethical Committee of Hangzhou First People’s Hospital. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 147 kb)
Rights and permissions
About this article
Cite this article
Lu, L., Liu, B., Leng, J. et al. Electrochemical mixed aptamer-antibody sandwich assay for mucin protein 16 detection through hybridization chain reaction amplification. Anal Bioanal Chem 412, 7169–7178 (2020). https://doi.org/10.1007/s00216-020-02849-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02849-5