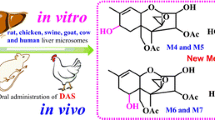
Abstract
Ochratoxin A (OTA) is a mycotoxin that frequently contaminates a wide variety of food and feedstuffs. The metabolism of OTA greatly affects fate and toxicity in humans and animals, because of its possible carcinogenic character (International Agency for Research on Cancer (IARC), group 2B). To completely characterize the metabolites of OTA, the metabolism of OTA in liver microsomes of rats, chickens, swine, goats, cows, and humans was investigated using ultra-performance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry (UPLC-Q/TOF-MS). In addition, an in vivo comparative metabolism study of OTA was performed among rats and chickens after oral administration of OTA. As a result, a clear metabolic profile of OTA in different species was proposed, and a total of eight metabolites were identified, of which three hydroxylated metabolites at the phenylalanine moiety were discovered for the first time (preliminarily identified as 9′-OH-OTA, 7′-OH-OTA, and 5′-OH-OTA). Considerable amounts of 7′-OH-OTA were detected in different species’ liver microsomes, especially in chickens and humans. Moreover, the metabolism of OTA in chickens was elucidated for the first time in the present study. The 7′-OH-OTA proved to be the main metabolite in vitro and in vivo in chickens. Furthermore, the 4(S)-OH-OTA isomer was the major one, and 4(R)-OH-OTA the minor metabolite in chickens, which was different from others where 4R was the major. OTA undergoes metabolism via three different pathways, namely hydroxylation, dechlorination, and conjugation. The proposed metabolic pathways of OTA in various species provide the scientific community useful data for the toxicological safety evaluation of OTA among different species, and will further facilitate the food safety evaluation of OTA.

In Vitro and in Vivo Metabolism of Ochratoxin A: A Comparative Study Using Ultra-Performance Liquid Chromatography-Quadrupole/Time-of-Flight Hybrid Mass Spectrometry







Similar content being viewed by others
References
Zheng RS, Xu H, Wang WL, Zhan RT, Chen WW (2014) Simultaneous determination of aflatoxin B-1, B-2, G(1), G(2), ochratoxin A, and sterigmatocystin in traditional Chinese medicines by LC-MS-MS. Anal Bioanal Chem 406:3031–3039
Varga E, Glauner T, Berthiller F, Krska R, Schuhmacher R, Sulyok M (2013) Development and validation of a (semi-)quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal Bioanal Chem 405:5087–5104
Wu Q, Dohnal V, Huang L, Kuca K, Wang X, Chen G, Yuan Z (2011) Metabolic pathways of ochratoxin A. Curr Drug Metab 12:1–10
Solfrizzo M, Gambacorta L, Lattanzio VM, Powers S, Visconti A (2011) Simultaneous LC-MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, alpha and beta-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal Bioanal Chem 401:2831–2841
Reiter EV, Cichna-Markl M, Chung DH, Shim WB, Zentek J, Razzazi-Fazeli E (2011) Determination of ochratoxin A in grains by immuno-ultrafiltration and HPLC-fluorescence detection after postcolumn derivatisation in an electrochemical cell. Anal Bioanal Chem 400:2615–2622
Shephard GS, Burger HM, Gambacorta L, Krska R, Powers SP, Rheeder JP, Solfrizzo M, Sulyok M, Visconti A, Warth B, van der Westhuizen L (2013) Mycological analysis and multimycotoxins in maize from rural subsistence farmers in the former Transkei, South Africa. J Agric Food Chem 61:8232–8240
Wang HW, Wang JQ, Zheng BQ, Li SL, Zhang YD, Li FD, Zheng N (2014) Cytotoxicity induced by ochratoxin A, zearalenone, and alpha-zearalenol: effects of individual and combined treatment. Food Chem Toxicol. doi:10.1016/j.fct.2014.05.032
Chopra M, Link P, Michels C, Schrenk D (2010) Characterization of ochratoxin A-induced apoptosis in primary rat hepatocytes. Cell Biol Toxicol 26:239–254
Trucksess MW, Scott PM (2008) Mycotoxins in botanicals and dried fruits: a review. Food Addit Contam A 25:181–192
Ringot D, Chango A, Schneider YJ, Larondelle Y (2006) Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem Biol Interact 159:18–46
Vukelic M, Sostaric B, Belicza M (1992) Pathomorphology of Balkan endemic nephropathy. Food Chem Toxicol 30:193–200
Han Z, Zhao Z, Shi J, Liao Y, Zhang D, Wu Y, De Saeger S, Wu A (2013) Combinatorial approach of LC-MS/MS and LC-TOF-MS for uncovering in vivo kinetics and biotransformation of ochratoxin A in rat. J Chromatogr B 925:46–53
Suzuki S, Satoh T, Yamazaki M (1977) The pharmacokinetics of ochratoxin A in rats. Jpn J Pharmacol 27:735–744
Hansen CE, Dueland S, Drevon CA, Stormer FC (1982) Metabolism of ochratoxin A by primary cultures of rat hepatocytes. Appl Environ Microbiol 43:1267–1271
Stormer FC, Pedersen JI (1980) Formation of 4-hydroxyochratoxin A from ochratoxin A by rat liver microsomes. Appl Environ Microbiol 39:971–975
Stormer FC, Hansen CE, Pedersen JI, Hvistendahl G, Aasen AJ (1981) Formation of (4R)- and (4S)-4-hydroxyochratoxin A from ochratoxin A by liver microsomes from various species. Appl Environ Microbiol 42:1051–1056
Stormer FC, Storen O, Hansen CE, Pedersen JI, Aasen AJ (1983) Formation of (4R)- and (4S)-4-hydroxyochratoxin A and 10-hydroxyochratoxin A from Ochratoxin A by rabbit liver microsomes. Appl Environ Microbiol 45:1183–1187
Mally A, Keim-Heusler H, Amberg A, Kurz M, Zepnik H, Mantle P, Volkel W, Hard GC, Dekant W (2005) Biotransformation and nephrotoxicity of ochratoxin B in rats. Toxicol Appl Pharmacol 206:43–53
Li S, Marquardt RR, Frohlich AA (2000) Identification of ochratoxins and some of their metabolites in bile and urine of rats. Food Chem Toxicol 38:141–152
**ao H, Madhyastha S, Marquardt RR, Li S, Vodela JK, Frohlich AA, Kemppainen BW (1996) Toxicity of ochratoxin A, its opened lactone form and several of its analogs: structure-activity relationships. Toxicol Appl Pharmacol 137:182–192
Zepnik H, Volkel W, Dekant W (2003) Metabolism and toxicokinetics of the mycotoxin ochratoxin A in F344 rats. Mycotoxin Res 19:102–107
Dai J, Park G, Wright MW, Adams M, Akman SA, Manderville RA (2002) Detection and characterization of a glutathione conjugate of ochratoxin A. Chem Res Toxicol 15:1581–1588
Hohler D, Sudekum KH, Wolffram S, Frohlich AA, Marquardt RR (1999) Metabolism and excretion of ochratoxin A fed to sheep. J Anim Sci 77:1217–1223
Kiessling KH, Pettersson H, Sandholm K, Olsen M (1984) Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl Environ Microbiol 47:1070–1073
Nip WK, Chu FS (1979) Fate of ochratoxin A in goats. J Environ Sci Health B 14:319–333
Yang S, Shi W, Hu D, Zhang S, Zhang H, Wang Z, Cheng L, Sun F, Shen J, Cao X (2014) In vitro and in vivo metabolite profiling of valnemulin using ultraperformance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. J Agric Food Chem 62:9201–9210
Yang S, Li Y, Cao X, Hu D, Wang Z, Wang Y, Shen J, Zhang S (2013) Metabolic pathways of T-2 toxin in in vivo and in vitro systems of Wistar rats. J Agric Food Chem 61:9734–9743
Wu H, Li L, Shen J, Wang Y, Liu K, Zhang S (2012) In vitro metabolism of cyadox in rat, chicken and swine using ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal 67–68:175–185
Shen J, Yang C, Wu C, Feng P, Wang Z, Li Y, Zhang S (2010) Identification of the major metabolites of quinocetone in swine urine using ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun Mass Spectrom 24:375–383
Ivanova L, Faeste CK, Uhlig S (2011) In vitro phase I metabolism of the depsipeptide enniatin B. Anal Bioanal Chem 400:2889–2901
Arnhard K, Gottschall A, Pitterl F, Oberacher H (2014) Applying ‘Sequential Windowed Acquisition of All Theoretical Fragment Ion Mass Spectra’ (SWATH) for systematic toxicological analysis with liquid chromatography-high-resolution tandem mass spectrometry. Anal Bioanal Chem. doi:10.1007/s00216-014-8262-1
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Pearce RE, McIntyre CJ, Madan A, Sanzgiri U, Draper AJ, Bullock PL, Cook DC, Burton LA, Latham J, Nevins C, Parkinson A (1996) Effects of freezing, thawing, and storing human liver microsomes on cytochrome P450 activity. Arch Biochem Biophys 331:145–169
Han Z, Tangni EK, Di Mavungu JD, Vanhaecke L, De Saeger S, Wu A, Callebaut A (2013) In vitro glucuronidation of ochratoxin a by rat liver microsomes. Toxins 5:2671–2685
Storen O, Holm H, Stormer FC (1982) Metabolism of ochratoxin A by rats. Appl Environ Microbiol 44:785–789
El Adlouni C, Pinelli E, Azemar B, Zaoui D, Beaune P, Pfohl-Leszkowicz A (2000) Phenobarbital increases DNA adduct and metabolites formed by ochratoxin A: role of CYP 2C9 and microsomal glutathione-S-transferase. Anal Bioanal Chem 35:123–131
Acknowledgments
This work was supported by grants from the International Science & Technology Cooperation Program of China (2012DFG31840) and the National Natural Science Foundation of China (No. U1301214 and 31372475). Furthermore, the authors would like to acknowledge **aoli Song, Chenglong Li, Ying Wang, Haixia Wu, Kaili Liu, and Lu Zhang for their practical work.
Conflict of interest
The authors declare no conflicting financial interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Shupeng Yang and Huiyan Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 310 kb)
Rights and permissions
About this article
Cite this article
Yang, S., Zhang, H., De Saeger, S. et al. In vitro and in vivo metabolism of ochratoxin A: a comparative study using ultra-performance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. Anal Bioanal Chem 407, 3579–3589 (2015). https://doi.org/10.1007/s00216-015-8570-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8570-0