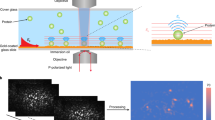
Abstract
Determination of protein surface excess is an important way of evaluating the properties of biomaterials and the characteristics of biosensors. A single-molecule counting method is presented that uses a standard fluorescence microscope to measure coverage of a liquid/solid interface by adsorbed proteins. The extremely low surface excess of lysozyme and bovine serum albumin (BSA), in a bulk concentration range from 0.3 nmol L−1 (0.02 μg mL−1) to 3 nmol L−1 (0.2 μg mL−1), were measured by recording the counts of spatially isolated single molecules on either hydrophilic (glass) or hydrophobic (polydimethylsiloxane, PDMS) surfaces at different pH. The differences observed in amounts of adsorbed proteins under different experimental conditions can be qualitatively explained by the combined interactions of electrostatic and hydrophobic forces. This, in turn, implies that single-molecule counting is an effective way of measuring surface coverage at a liquid/solid interface.

Adsorption fraction of proteins on different surfaces changed with pH.




Similar content being viewed by others
References
Nakanishi K, Sakiyama T, Imamura K (2001) J Biosci Bioeng 91:233–244
Cohen AE, Moerner WE (2006) Proc Natl Acad Sci USA 103:4362–4365
Yu J, **a J, Ren XJ, Lao KQ, **e XS (2006) Science 311:1600–1603
Bates M, Huang B, Dempsey GT, Zhuang XW (2007) Science 317:1749–1753
Dickson RM, Norris DJ, Tzeng YL, Moerner WE (1996) Science 274:966–969
Gai HW, Li Y, Silber-Li ZH, Ma YF, Lin BC (2005) Lab Chip 5:443–449
Gai HW, Stayton I, Liu X, Lin BC, Ma YF (2007) Trends Anal Chem 26:980–992
Roach P, Farrar D, Perry CC (2005) J Am Chem Soc 127:8168–8173
Roach P, Farrar D, Perry CC (2006) J Am Chem Soc 128:3939–3945
Glomm WR, Halskau O, Hanneseth AMD, Volden S (2007) J Phys Chem B 111:14329–14345
McClellan SJ, Franses EI (2005) Colloids Surf A 260:265–275
Kim G, Gurau M, Kim J, Cremer PS (2002) Langmuir 18:2807–2811
Su TJ, Lu JR, Thomas RK, Cui ZF (1999) J Phys Chem B 103:3727–3736
Lu JR, Su TJ, Howlin BJ (1999) J Phys Chem B 103:5903–5909
Su TJ, Lu JR, Thomas RK, Cui ZF, Penfold J (1998) J Phys Chem B 102:8100–8108
Su TJ, Lu JR, Thomas RK, Cui ZF, Penfold J (1998) Langmuir 14:438–445
Lu JR, Su TJ, Thirtle PN, Thomas RK, Rennie AR, Cubitt R (1998) J Colloid Interf Sci 206:212–223
McGuire J, Wahlgren MC, Arnebrant T (1995) J Colloid Interf Sci 170:182–192
Yeung ES (2004) Annu Rev Phys Chem 55:97–126
Xu XHN, Yeung ES (1998) Science 281:1650–1653
Kang SH, Yeung ES (2002) Anal Chem 74:6334–6339
Fang N, Zhang H, Li JW, Li HW, Yeung ES (2007) Anal Chem 79:6047–6054
Kwok KC, Yeung KM, Cheung NH (2007) Langmuir 23:1948–1952
Li L, Tian XZ, Zou GZ, Shi ZK, Zhang XL, ** WR (2008) Anal Chem 80:3999–4006
Gai HW, Griess GA, Demeler B, Weintraub ST, Serwer P (2007) J Microscopy 226:256–262
Unger M, Kartalov E, Chiu CS, Lester HA, Quake SR (1999) Biotechniques 27:1008–1009
Salim M, O'Sullivan B, McArthur SL, Wright PC (2007) Lab Chip 7:64–70
Janasek D, Franzke J, Manz A (2006) Nature 442:374–380
Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung WY, Haugland RP (1999) J Histochem Cytochem 47:1179–1188
Han R, Zhang YW, Dong XL, Gai HW, Yeung ES (2008) Anal Chim Acta 619:209–214
Gai HW, Wang Q, Ma YF, Lin BC (2005) Angew Chem Int Ed 44:5107–5110
Su TJ, Lu JR, Thomas RK, Cui ZF, Penfold J (1998) J Colloid Interf Sci 203:419–429
Cardamone M, Puri NK (1992) Biochem J 282:589–593
Israelachvili J, Pashley R (1982) Nature 300:341–342
van Oss CJ (2003) J Mol Recognit 16:177–190
Menon MK, Zydney AL (1998) Anal Chem 70:1581–1584
Bohme U, Scheler U (2007) Chem Phys Lett 435:342–345
Larsen AE, Grier DG (1997) Nature 385:230–233
Claesson PM, Blomberg E, Froberg JC, Nylander T, Arnebrant T (1995) Adv Colloid Interf Sci 57:161–227
Buijs J, Hlady V (1997) J Colloid Interf Sci 190:171–181
Hillborg H, Tomczak N, Olah A, Schonherr H, Vancso GJ (2004) Langmuir 20:785–794
** MH, Feng XJ, ** JM, Zhai J, Cho KW, Feng L, Jiang L (2005) Macromol Rapid Commun 26:1805–1809
Wu DP, Luo Y, Zhou XM, Dai ZP, Lin BC (2005) Electrophoresis 26:211–218
Wu DP, Zhao BX, Dai ZP, Qin JH, Lin BC (2006) Lab Chip 6:942–947
Ocvirk G, Munroe M, Tang T, Oleschuk R, Westra K, Harrison DJ (2000) Electrophoresis 21:107–115
Spehar AM, Koster S, Linder V, Kulmala S, de Rooij NF, Verpoorte E, Sigrist H, Thormann W (2003) Electrophoresis 24:3674–3678
Tsutsui T, Lichan E, Nakai S (1986) J Food Sci 51:1268–1272
Acknowledgements
The authors are grateful to the Natural Science Foundation of China (NSFC, 20705007, 30570479, 30670532) and the Hunan University “985” Fund for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, N., Tang, H., Gai, H. et al. Determination of protein surface excess on a liquid/solid interface by single-molecule counting. Anal Bioanal Chem 394, 1879–1885 (2009). https://doi.org/10.1007/s00216-009-2888-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-2888-4