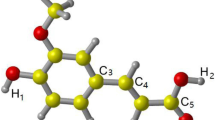
Abstract
Avenanthramides (AVs) are the phytochemicals found in cereals exclusively in oats. These are widely known natural substances that have antioxidant properties. The free radical deactivation potential of the eight AVs against five reactive species has been studied in physiological pH. At physiological pH, the radical quenching processes were studied using the sequential proton loss followed by electron transfer (SPLET) from the phenolic hydroxyl groups. Using density functional theory (DFT) computations, theoretical studies have been carried out in the gas phase and aqueous solution at M06-2X/6-31 + G (d,p) level of theory. The free radical scavenging ability of the studied AVs was analyzed by using conceptual density functional theory-based parameters and electrostatic potential analysis. By examining the hydrogen atom and electron affinities of each reactive species, the relative destructive potential of each has been compared. The electron transfer capabilities between the studied compound and reactive species were identified by utilizing the ionization energy and electron affinity plots. Additionally, by calculating the redox potentials and equilibrium constants for the entire process in the aqueous solution, the viability of scavenging the free radical species by selected AVs (both in neutral and mono-deprotonated) has been investigated. From the analysis, the neutral as well as the mono-deprotonated form of AVs are found to scavenge •OH and •OOH, and •NO2 radicals effectively, while they are inefficacious toward the O2•‾ and •NO radicals.
Graphical Abstract







Similar content being viewed by others
Data availability
Not applicable.
References
Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14:111–129. https://doi.org/10.1038/nrd4510
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83:770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Dehelean CA, Marcovici I, Soica C, et al (2021) Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules 26
Rajan VK, Shameera Ahamed TK, Muraleedharan K (2018) Studies on the UV filtering and radical scavenging capacity of the bitter masking flavanone Eriodictyol. J Photochem Photobiol B Biol 185:254–261. https://doi.org/10.1016/j.jphotobiol.2018.06.017
Rajan VK, Muraleedharan K (2017) A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, Gallic acid. Food Chem 220:93–99. https://doi.org/10.1016/j.foodchem.2016.09.178
Sumayya PC, Mujeeb VMA, Muraleedharan K (2022) Radical scavenging capacity, UV activity, and molecular docking studies of 2ʹ, 5ʹ, 3, 4-Tetrahydroxychalcone: An insight into the photoprotection. Chem Phys Impact 5:100126. https://doi.org/10.1016/j.chphi.2022.100126
Shameera Ahamed TK, Rajan VK, Sabira K, Muraleedharan K (2019) DFT and QTAIM based investigation on the structure and antioxidant behavior of lichen substances Atranorin, Evernic acid and Diffractaic acid. Comput Biol Chem 80:66–78. https://doi.org/10.1016/j.compbiolchem.2019.03.009
Ragi C, Muraleedharan K (2023) Antioxidant activity of Hibiscetin and Hibiscitrin: insight from DFT, NCI, and QTAIM. Theor Chem Acc 142:30. https://doi.org/10.1007/s00214-023-02970-5
Grzesik M, Naparło K, Bartosz G, Sadowska-Bartosz I (2018) Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem 241:480–492. https://doi.org/10.1016/j.foodchem.2017.08.117
Pietta P-G (2000) Flavonoids as Antioxidants. J Nat Prod 63:1035–1042. https://doi.org/10.1021/np9904509
Rajan VK, Ragi C, Muraleedharan K (2019) A computational exploration into the structure, antioxidant capacity, toxicity and drug-like activity of the anthocyanidin “Petunidin.” Heliyon 5:e02115. https://doi.org/10.1016/j.heliyon.2019.e02115
Rajan VK, Hasna CK, Muraleedharan K (2018) The natural food colorant Peonidin from cranberries as a potential radical scavenger—A DFT based mechanistic analysis. Food Chem 262:184–190. https://doi.org/10.1016/j.foodchem.2018.04.074
Pottachola S, Kaniyantavida A, Karuvanthodiyil M (2021) DFT study of structure and radical scavenging activity of natural pigment delphinidin and derivatives. In: Glossman-Mitnik D (ed). IntechOpen, Rijeka, p Ch. 16
Borges Bubols G, da Rocha VD, Medina-Remon A et al (2013) The Antioxidant activity of coumarins and flavonoids. Mini-Reviews Med Chem 13:318–334
Hamadouche S, Ounissi A, Baira K et al (2021) Theoretical evaluation of the antioxidant activity of some stilbenes using the Density Functional Theory. J Mol Struct 1229:129496. https://doi.org/10.1016/j.molstruc.2020.129496
Gonzalez-Burgos E, Gomez-Serranillos MP (2012) Terpene compounds in nature: a review of their potential antioxidant activity. Curr Med Chem 19:5319–5341
Perrelli A, Goitre L, Salzano AM et al (2018) Biological activities, health benefits, and therapeutic properties of Avenanthramides: From skin protection to prevention and treatment of cerebrovascular diseases. Oxid Med Cell Longev 2018:6015351. https://doi.org/10.1155/2018/6015351
Li X, Zhou L, Yu Y et al (2022) The potential functions and mechanisms of oat on cancer prevention: A review. J Agric Food Chem 70:14588–14599. https://doi.org/10.1021/acs.jafc.2c06518
Yu Y, Zhou L, Li X et al (2022) The progress of nomenclature, structure, metabolism, and bioactivities of oat novel phytochemical: Avenanthramides. J Agric Food Chem 70:446–457. https://doi.org/10.1021/acs.jafc.1c05704
Peterson DM, Hahn MJ, Emmons CL (2002) Oat Avenanthramides exhibit antioxidant activities in vitro. Food Chem 79:473–478. https://doi.org/10.1016/S0308-8146(02)00219-4
Bratt K, Sunnerheim K, Bryngelsson S et al (2003) Avenanthramides in Oats (Avena sativa L.) and structure−antioxidant activity relationships. J Agric Food Chem 51:594–600. https://doi.org/10.1021/jf020544f
Fagerlund A, Sunnerheim K, Dimberg LH (2009) Radical-scavenging and antioxidant activity of Avenanthramides. Food Chem 113:550–556. https://doi.org/10.1016/j.foodchem.2008.07.101
Zhang T, Shao J, Gao Y et al (2017) Absorption and elimination of oat Avenanthramides in humans after acute consumption of oat cookies. Oxid Med Cell Longev 2017:2056705. https://doi.org/10.1155/2017/2056705
Dimberg LH, Theander O, Lingnert H (1993) Avenanthramides–a group of phenolic antioxidants in oats. Cereal Chem 70:637–641
Sumayya PC, Babu G, Muraleedharan K (2021) Quantum chemical investigation of the antiradical property of avenanthramides, oat phenolics. Heliyon 7:e06125. https://doi.org/10.1016/j.heliyon.2021.e06125
Xue Y, Teng Y, Chen M et al (2021) Antioxidant activity and mechanism of Avenanthramides: Double H+/e– processes and role of the catechol, guaiacyl, and carboxyl groups. J Agric Food Chem 69:7178–7189. https://doi.org/10.1021/acs.jafc.1c01591
Purushothaman A, Jishnu Gopal P, Janardanan D (2022) Mechanistic insights on the radical scavenging activity of oat avenanthramides. J Phys Org Chem 35:e4391. https://doi.org/10.1002/poc.4391
Pizzino G, Irrera N, Cucinotta M et al (2017) Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev 2017:8416763. https://doi.org/10.1155/2017/8416763
Sharifi-Rad M, Anil Kumar N V, Zucca P, et al. (2020) Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases . Front. Physiol. 11
Mellon I (2004) Transcription-coupled DNA repair, overview. In: Lennarz WJ, Lane MDBT-E of BC (eds). Elsevier, New York, pp 204–208
Islam BU, Habib S, Ahmad P et al (2015) Pathophysiological role of peroxynitrite induced DNA damage in human diseases: A special focus on Poly(ADP-ribose) Polymerase (PARP). Indian J Clin Biochem 30:368–385. https://doi.org/10.1007/s12291-014-0475-8
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. and DJF (2016) Gaussian 16, Revision C.01
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094. https://doi.org/10.1021/cr9904009
Analyzer AMW, Lu T (2019) Multiwfn. 7:
Humphrey W, Dalke A, Schulten K (1996) VMD: Visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Zhang J, Lu T (2021) Efficient evaluation of electrostatic potential with computerized optimized code. Phys Chem Chem Phys 23:20323–20328. https://doi.org/10.1039/D1CP02805G
Khlaifia D, Massuyeau F, Ewels C et al (2017) DFT modeling of novel donor-acceptor (D-A) molecules incorporating 3-hexylthiophene (3HT) for Bulk Heterojunction Solar Cells. ChemistrySelect 2:10082–10090. https://doi.org/10.1002/slct.201701481
Zhan C-G, Nichols JA, Dixon DA (2003) Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A 107:4184–4195. https://doi.org/10.1021/jp0225774
Putz MV (2013) Koopmans’ analysis of chemical hardness with spectral-like resolution. Sci World J 2013:348415. https://doi.org/10.1155/2013/348415
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity Index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Chakraborty D, Chattaraj PK (2021) Conceptual density functional theory based electronic structure principles. Chem Sci 12:6264–6279. https://doi.org/10.1039/d0sc07017c
Dutra FR, de Silva C, S, Custodio R (2021) On the accuracy of the direct method to calculate pKa from electronic structure calculations. J Phys Chem A 125:65–73. https://doi.org/10.1021/acs.jpca.0c08283
Kelly CP, Cramer CJ, Truhlar DG (2006) Aqueous solvation free energies of ions and ion−water clusters based on an accurate value for the absolute aqueous solvation free energy of the proton. J Phys Chem B 110:16066–16081. https://doi.org/10.1021/jp063552y
Mittal A, Vashistha VK, Das DK (2023) Free radical scavenging activity of Gallic acid toward various reactive oxygen, nitrogen, and Sulfur species: a DFT approach. Free Radic Res 1–10. https://doi.org/10.1080/10715762.2023.2197556
Coimbra M, Isacchi B, van Bloois L et al (2011) Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int J Pharm 416:433–442. https://doi.org/10.1016/j.ijpharm.2011.01.056
Abdel-Mottaleb MSA (2016) DFT studies of caffeic acid antioxidant: molecular orbitals and composite reactivity maps correlation with photophysical characteristics and photochemical stability. J Chem 2016:8727130. https://doi.org/10.1155/2016/8727130
Marković Z, Tošović J, Milenković D, Marković S (2016) Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput Theor Chem 1077:11–17. https://doi.org/10.1016/j.comptc.2015.09.007
Ferreira CA, Ni D, Rosenkrans ZT, Cai W (2018) Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res 11:4955–4984. https://doi.org/10.1007/s12274-018-2092-y
Alfadda AA, Sallam RM (2012) Reactive oxygen species in health and disease. J Biomed Biotechnol 2012:936486. https://doi.org/10.1155/2012/936486
Galano A (2015) Free radicals induced oxidative stress at a molecular level: The current status, challenges and perspectives of computational chemistry based protocols
Bartmess JE (1994) Thermodynamics of the Electron and the Proton. J Phys Chem 98:6420–6424. https://doi.org/10.1021/j100076a029
Najafi H (2013) Theoretical study of the substituent effects on the reaction enthalpies of the antioxidant mechanisms of stobadine derivatives in the gas-phase and water. J Theor Comput Chem 12:
Martínez A, Hernández-Marin E, Galano A (2012) Xanthones as antioxidants: A theoretical study on the thermodynamics and kinetics of the single electron transfer mechanism. Food Funct 3:442–450. https://doi.org/10.1039/C2FO10229C
Mittal A, Kakkar R (2020) The effect of solvent polarity on the antioxidant potential of echinatin, a retrochalcone, towards various ROS: a DFT thermodynamic study. Free Radic Res 54:777–786. https://doi.org/10.1080/10715762.2020.1849670
Acknowledgements
The author Sumayya P.C. expresses sincere gratitude to UGC-MANF for financial support (Research fellowship). The authors are also thankful to the Central Sophisticated Instrumentation Facility (CSIF) of the University of Calicut for the Gaussian 16 software support.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Author information
Authors and Affiliations
Contributions
S.P.C. involved in idea, methods, software, experiment setup, preparation of data and evaluation, and paper writing and editing. Dr. K.M. involved in monitoring, proof reading, final editing, and guidance.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Human and animal rights
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sumayya, P.C., Muraleedharan, K. Quenching of reactive species by Avenanthramides: theoretical insight to the thermodynamics of electron transfer. Theor Chem Acc 143, 37 (2024). https://doi.org/10.1007/s00214-024-03111-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-024-03111-2