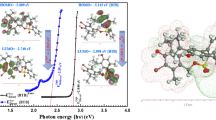
Abstract
DFT reactivity descriptors, the ultraviolet–visible spectra and hydrolysis mechanism of three cationic dyes [Malachite Green (MG), Brilliant Green (BG) and Ethyl Green (EG)] are performed with several exchange–correlation functional (global GGA, hybrids and range-separated). Using time-dependent density functional theory, the theoretical ultraviolet–visible absorption spectra of the three cationic dyes are obtained and obey the trend for the λmax: GGA > hybrid > range-separated functional. Thanks to the transition state theory, the barriers of hydrolysis mechanism of the cation structures dyes were obtained in gas and solution phase. It is shown that, for these systems the barriers are in order: BG+ < MG+ < EG2+ in gas and solution phase. In the two phases, the CAM-B3LYP functional gives the highest barriers and the M06 gives the lowest ones.












Similar content being viewed by others
References
Looney CW, Simpson WT (1954) Structures and π-electron spectra. III. Triphenylmethane dyes 1, 2, 3. J Am Chem Soc 76(24):6293–6300
Bendjabeur S, Zouaghi R, Zouchoune B, Sehili T (2018) DFT and TD-DFT insights, photolysis and photocatalysis investigation of three dyes with similar structure under UV irradiation with and without TiO2 as a catalyst: Effect of adsorption, pH and light intensity. Spectrochim Acta Part A Mol Biomol Spectrosc 190:494–505
Soto-Rojo R, Baldenebro-López J, Glossman-Mitnik D (2015) Study of chemical reactivity in relation to experimental parameters of efficiency in coumarin derivatives for dye sensitized solar cells using DFT. Phys Chem Chem Phys 17(21):14122–14129
Barker CC, Bride MH, Hallas G, Stamp A (1961) 249. Steric effects in di-and tri-arylmethanes. Part III. Electronic absorption spectra of derivatives of malachite green containing substituents in the phenyl ring. J Chem Soc 1285:1290
Engh RA, Petrich JW, Fleming GR (1985) Removal of coherent coupling artifact in ground-state recovery experiments: malachite green in water-methanol mixtures. J Phys Chem 89(4):618–621
Lian T, Locke B, Kholodenko Y, Hochstrasser RM (1994) Energy flow from solute to solvent probed by femtosecond IR spectroscopy: malachite green and heme protein solutions. J Phys Chem 98(45):11648–11656
Nagasawa Y, Ando Y, Okada T (1999) Solvent dependence of ultrafast ground state recovery of the triphenylmethane dyes, brilliant green and malachite green. Chem Phys Lett 312(2–4):161–168
Miyata R, Kimura Y, Terazima M (2002) Intermolecular energy transfer from the photo-excited molecule to solvent: Malachite Green. Chem Phys Lett 365(5–6):406–412
Sen P, Yamaguchi S, Tahara T (2010) Ultrafast dynamics of malachite green at the air/water interface studied by femtosecond time-resolved electronic sum frequency generation (TR-ESFG): an indicator for local viscosity. Faraday Discuss 145:411–428
Li G, Magana D, Dyer RB (2012) Direct observation and control of ultrafast photoinduced twisted intramolecular charge transfer (TICT) in triphenyl-methane dyes. J Phys Chem B 116(41):12590–12596
Yang J, Yang X, Lin Y, Ng TB, Lin J, Ye X (2015) Laccase-catalyzed decolorization of malachite green: performance optimization and degradation mechanism. PLoS ONE 10(5):e0127714
Wang J et al (2012) Pathway and molecular mechanisms for malachite green biodegradation in Exiguobacterium sp. MG2. PLoS ONE 7(12):e51808
Navarro P, Zapata JP, Gotor G, Gonzalez-Olmos R, Gómez-López VM (2019) Degradation of malachite green by a pulsed light/H2O2 process. Water Sci Technol 79(2):260–269
Guillaumont D, Nakamura S (2000) Calculation of the absorption wavelength of dyes using time-dependent density-functional theory (TD-DFT). Dye Pigment 46(2):85–92
Preat J, Jacquemin D, Wathelet V, André J-M, Perpète EA (2007) Towards the understanding of the chromatic behaviour of triphenylmethane derivatives. Chem Phys 335(2–3):177–186
Rafiq S, Yadav R, Sen P (2010) Microviscosity inside a nanocavity: a femtosecond fluorescence up-conversion study of malachite green. J Phys Chem B 114(44):13988–13994
Nakayama A, Taketsugu T (2011) Ultrafast nonradiative decay of electronically excited states of malachite green: ab initio calculations. J Phys Chem A 115(32):8808–8815
** dynamics simulations of malachite green: a triphenylmethane dye. J Phys Chem A 119(22):5607–5617
Fox BM, Hallas G, Hepworth JD, Mason D (1980) The hydrolysis of brilliant green and some derivatives. I. A kinetic and equilibrium study of the hydrolysis of brilliant green. J Chem Technol Biotechnol 30(1):317–323
Beach SF, Hepworth JD, Mason D, Swarbrick EA (1999) A kinetic study of the hydrolysis of crystal violet and some terminal and bridged analogues. Dye Pigment 42(1):71–77
Frisch MJ (2009) Gaussian 09, version A. 1. Gaussian Inc., Pittsburgh
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 33(12):8822
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58(8):1200–1211
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor Chem Acc 120(1):215–241
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2(2):364–382
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110(13):6158–6170
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393(1–3):51–57
Chai J-D, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128(8):84106
Hehre WJ, Ditchfield R, Pople JA (1972) Self—consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56(5):2257–2261
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24(6):669–681
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102(11):1995–2001
Improta R (2012) Uv–visible absorption and emission energies in condensed phase by PCM/TD-DFT methods. In: Computational strategies for spectroscopy: from small molecules to nano systems. Wiley, Chichester, pp 39–76
Kawauchi S, Antonov L, Okuno Y (2014) Prediction of the color of dyes by using time-dependent density functional theory (TD-DFT). Bulg Chem Commun 46:228–237
Frisch MJ et al. (2016) Gaussian 16 revision a. 03. 2016; Gaussian inc. Wallingford, CT, vol. 2, no. 4.
Le Bahers T, Adamo C, Ciofini I (2011) A qualitative index of spatial extent in charge-transfer excitations. J Chem Theory Comput 7(8):2498–2506
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor-acceptor perspective. Cambridge University Press, Cambridge
Andersen P (1965) An electron diffraction investigation of triphenylmethane in gas phase. ACTA Chem Scand 19(3):622
Andersen P, Klewe B (1967) The crystal structure of the free radical tri-p-nitrophenylmethyl. Acta Chem Scand 21(10):2
Lister DG, Tyler JK, Høg JH, Larsen NW (1974) The microwave spectrum, structure and dipole moment of aniline. J Mol Struct 23(2):253–264
Fukuyo M, Hirotsu K, Higuchi T (1982) The structure of aniline at 252 K. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem 38(2):640–643
Kreutzer J, Blaha P, Schubert U (2016) Assessment of different basis sets and DFT functionals for the calculation of structural parameters, vibrational modes and ligand binding energies of Zr4O2 (carboxylate) 12 clusters. Comput Theor Chem 1084:162–168
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comput Chem 20(1):129–154
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105(26):7512–7516
Martínez J (2009) Local reactivity descriptors from degenerate frontier molecular orbitals. Chem Phys Lett 478(4–6):310–322
El Haouti R et al (2019) Cationic dyes adsorption by Na-montmorillonite nano clay: experimental study combined with a theoretical investigation using DFT-based descriptors and molecular dynamics simulations. J Mol Liq 290:111139
Jacquemin D, Assfeld X, Preat J, Perpète EA (2007) Comparison of theoretical approaches for predicting the UV/Vis spectra of anthraquinones. Mol Phys 105(2–3):325–331
Perpète EA, Lambert C, Wathelet V, Preat J, Jacquemin D (2007) Ab initio studies of the λmax of naphthoquinones dyes. Spectrochim Acta Part A Mol Biomol Spectrosc 68(5):1326–1333
Jacquemin D, Preat J, Wathelet V, Fontaine M, Perpète EA (2006) Thioindigo dyes: highly accurate visible spectra with TD-DFT. J Am Chem Soc 128(6):2072–2083
Jacquemin D, Preat J, Wathelet V, Perpète EA (2006) Substitution and chemical environment effects on the absorption spectrum of indigo. J Chem Phys 124(7):74104
Perpète EA, Preat J, André J-M, Jacquemin D (2006) An ab initio study of the absorption spectra of indirubin, isoindigo, and related derivatives. J Phys Chem A 110(17):5629–5635
Jacquemin D et al (2006) Time-dependent density functional theory investigation of the absorption, fluorescence, and phosphorescence spectra of solvated coumarins. J Chem Phys 125(16):164324
Perpète EA, Maurel F, Jacquemin D (2007) TD−DFT investigation of diarylethene dyes with cyclopentene, dihydrothiophene, and dihydropyrrole bridges. J Phys Chem A 111(25):5528–5535
Jacquemin D, Perpete EA, Scuseria GE, Ciofini I, Adamo C (2008) TD-DFT performance for the visible absorption spectra of organic dyes: conventional versus long-range hybrids. J Chem Theory Comput 4(1):123–135
Jacquemin D, Perpète EA, Scalmani G, Frisch MJ, Kobayashi R, Adamo C (2007) Assessment of the efficiency of long-range corrected functionals for some properties of large compounds. J Chem Phys 126(14):144105
Jacquemin D, Preat J, Charlot M, Wathelet V, André J-M, Perpète EA (2004) Theoretical investigation of substituted anthraquinone dyes. J Chem Phys 121(4):1736–1743
Perpète EA, Wathelet V, Preat J, Lambert C, Jacquemin D (2006) Toward a theoretical quantitative estimation of the λmax of anthraquinones-based dyes. J Chem Theory Comput 2(2):434–440
El Kassimi A, Boutouil A, El Himri M, Laamari MR, El Haddad M (2020) Selective and competitive removal of three basic dyes from single, binary and ternary systems in aqueous solutions: a combined experimental and theoretical study. J Saudi Chem Soc 24(7):527–544
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3(5):1486–1494
Savin A, Silvi B, Colonna F (1996) Topological analysis of the electron localization function applied to delocalized bonds. Can J Chem 74(6):1088–1096
Savin A, Nesper R, Wengert S, Fässler TF (1997) ELF: the electron localization function. Angew Chemie Int Ed English 36(17):1808–1832
Chamorro E, Pérez P, Domingo LR (2013) On the nature of Parr functions to predict the most reactive sites along organic polar reactions. Chem Phys Lett 582:141–143. https://doi.org/10.1016/j.cplett.2013.07.020
Chamorro E, Pérez P (2005) Condensed-to-atoms electronic Fukui functions within the framework of spin-polarized density-functional theory. J Chem Phys 123(11):114107
Galván M, Vela A, Gázquez JL (1988) Chemical reactivity in spin-polarized density functional theory. J Phys Chem 92(22):6470–6474. https://doi.org/10.1021/j100333a056
Morell C, Grand A, Toro-Labbe A (2005) New dual descriptor for chemical reactivity. J Phys Chem A 109(1):205–212
Morell C, Grand A, Toro-Labbé A (2006) Theoretical support for using the Δf (r) descriptor. Chem Phys Lett 425(4–6):342–346
Abdoul-Carime H, et al. (2014). Velocity of a molecule evaporated from a water nanodroplet: Maxwell–Boltzmann statistics versus non-ergodic events (Angew. Chem. Int. Ed. 54, 14685–14689). Angew. Chem. Int. Ed.
Mechachti F, Lakehal S, Lakehal A, Morell C, Merzoud L, Chermette H (2021) Predicted structure and selectivity of 3d transition metal complexes with glutamic N, N-bis (carboxymethyl) acid. New J Chem 45(39):18366–18378
Stoyanov ES, Stoyanova IV, Reed CA (2010) The structure of the hydrogen ion (Haq+) in water. J Am Chem Soc 132(5):1484–1485
Berthias F et al (2015) Proton migration in clusters consisting of protonated pyridine solvated by water molecules. ChemPhysChem 16(15):3151–3155
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113(18):6378–6396
Schuster P (1976) In the hydrogen bond. In: Schuster P, Zundel G, Sandorfy C (eds) vol 2. North-Holland, Amsterdam
Eigen M (1964) Proton transfer, acid-base catalysis, and enzymatic hydrolysis. Part I: elementary processes. Angew Chemie Int Ed English 3(1):1–19
Agmon N (1996) Hydrogen bonds, water rotation and proton mobility. J Chim Phys Phys Chim Biol 93:1714–1736
Walrafen GE, Fisher MR, Hokmabadi MS, Yang W (1986) Temperature dependence of the low-and high-frequency Raman scattering from liquid water. J Chem Phys 85(12):6970–6982
Carey DM, Korenowski GM (1998) Measurement of the Raman spectrum of liquid water. J Chem Phys 108(7):2669–2675
Reed CA (2013) Myths about the proton. The nature of H+ in condensed media. Acc Chem Res 46(11):2567–2575
Śmiechowski M, Stangret J (2006) Proton hydration in aqueous solution: Fourier transform infrared studies of HDO spectra. J Chem Phys 125(20):204508
Rees DC, Lipscomb WN (1982) Refined crystal structure of the potato inhibitor complex of carboxypeptidase A at 2.5 Å resolution. J Mol Biol 160(3):475–498
Lamine W et al (2018) Unexpected structure of a Helical N4-Schiff-base Zn (II) complex and its demetallation: experimental and theoretical studies. ChemPhysChem 19(21):2938–2946
De Grotthuss CJT (2006) Memoir on the decomposition of water and of the bodies that it holds in solution by means of galvanic electricity. Biochim Biophys Acta (BBA) Bioenerg 1757(8):871–875
Marx D (2006) Proton transfer 200 years after von Grotthuss: insights from ab initio simulations. ChemPhysChem 7(9):1848–1870
Tuckerman M, Laasonen K, Sprik M, Parrinello M (1995) Ab initio molecular dynamics simulation of the solvation and transport of hydronium and hydroxyl ions in water. J Chem Phys 103(1):150–161
Mardirossian N, Head-Gordon M (2017) Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals. Mol Phys 115(19):2315–2372
Chan B, Gilbert ATB, Gill PMW, Radom L (2014) Performance of density functional theory procedures for the calculation of proton-exchange barriers: unusual behavior of M06-type functionals. J Chem Theory Comput 10(9):3777–3783
Karton A, O’Reilly RJ, Radom L (2012) Assessment of theoretical procedures for calculating barrier heights for a diverse set of water-catalyzed proton-transfer reactions. J Phys Chem A 116(16):4211–4221
Karton A, O’Reilly RJ, Chan B, Radom L (2012) Determination of barrier heights for proton exchange in small water, ammonia, and hydrogen fluoride clusters with G4 (MP2)-type, MPn, and SCS-MPn procedures. A Caveat. J Chem Theory Comput 8(9):3128–3136
Acknowledgements
NL acknowledges the financial support of the Algerian Ministry of Higher Education and Scientific Research and the DGRSDT (Research Project B00L01UN280120220002). The GENCI/CINES (Project cpt2130) and the PSMN of the ENS-Lyon are acknowledged for HPC resources/computer time. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
DT, NL, and HC contributed to conceptualization, methodology, and investigation. NL and HC performed writing—original draft preparation. CM, LM, NL, and HC performed writing—review and editing. DT, NL, HM, and BW performed experimental measurements. NM, NO, and CM provided resources. DT, NL, LM, and HC performed data curation. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taharchaouche, D., Latelli, N., Merouani, H. et al. Degradation by hydrolysis of three triphenylmethane dyes: DFT and TD-DFT study. Theor Chem Acc 142, 10 (2023). https://doi.org/10.1007/s00214-022-02950-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-022-02950-1