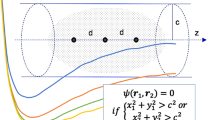
Abstract
The energy of an atomic or molecular system undergoing Coulomb interactions is well known at the integer numbers of its neutral or ionic configurations. Nevertheless, the physical domains (atoms in molecules) inside the whole molecular system possess a non-integer number of particles due to the electron exchange with its surrounding. Hence, the dependence of the energy, the density matrix and their marginal distributions (reduced density matrices) with the number of particles become a problem of fundamental importance in the description of the electron distribution, its properties and transformations. In this work, we present a rigorous mathematical and physical basis for the treatment of this problem within the grand-canonical statistical distribution of few particles. In this context, the derivatives of the energy and the density referred as chemical descriptors (especially chemical potential and hardness) are analyzed in both cases, when the system is isolated and when it is subject to the interaction with an environment. The ground state energy convexity dependence with the number of particles of these systems simplifies the description.
Similar content being viewed by others
References
Szabo A, Ostlund NS (1989) Modern quantum chemistry. Introduction to advanced electronic structure theory. Dover Publications Inc, Mineloa, New York
Helgaker T, Jörgensen P, Olsen J (2000) Molecular electronic structure theory. Wiley Ltd, Chitester
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103(5):1793–1873
Perdew JP, Parr RG, Levy M, Balduz J Jr (1982) Density-functional theory for fractional particle number: derivative discontinuities of the energy. Phys Rev Lett 49(23):1691–1694
Perdew JP (1985) In: Dreizler RM, da Providencia J (eds) Density functional theory methods in physics, NATO ASI series. Plenum, New York
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford Univesity Press, New York
Iczkowski RP, Margrave JL (1961) Electronegativity. J Am Chem Soc 83(17):3547–3551
Morales J, Martinez TJ (2001) Classical fluctuating charge theories: the maximum entropy valence bond formalism and relationships to previous models. J Phys Chem A 105(12):2842–2850
Ayers PW, Parr RG (2008) Local hardness equalization: exploiting the ambiguity. J Chem Phys 128(18):184108
Parr RG, Pariser R (2013) In: Ghosh SK, Chattaraj PK (eds) Concepts and methods in modern theoretical chemistry: electronic structure and reactivity. CRC Press, Boca Raton
Valone SM (2011) A concept of fragment hardness, independent of net charge, from a wave-function perspective. J Phys Chem Lett 2(20):2618–2622 and references
Bochicchio RC, Rial D (2012) Energy convexity and density matrices in molecular systems. J Chem Phys 137(22):226101
Bochicchio RC, Miranda-Quintana RA, Rial D (2013) Reduced density matrices in molecular systems: grand-canonical electron states. J Chem Phys 139(19):199101
Nalewajski RF (1998) Kohn–Sham description of equilibria and charge transfer in reactive systems. Int J Quant Chem 69(4):591–605
Valone SM, Atlas SR (2006) Energy dependence on fractional charge for strongly interacting subsystems. Phys Rev Lett 97(25):256402
Bochicchio RC, Lain L, Torre A (2003) Atomic valence in molecular systems. Chem Phys Lett 375(1–2):45–53 and references
Lain L, Torre A, Bochicchio RC (2004) Studies of population analysis at the correlated level: determination of three-center bond indices. J Phys Chem A 108(18):4132–4137 and references
Bader RFW (1994) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Popelier P (1999) Atoms in molecules: an introduction. Pearson Edu, London
Yang WT, Zhang YK, Ayers PW (2000) Degenerate ground states and fractional number of electrons in density and reduced density matrix functional theory. Phys Rev Lett 84(22):5172–5175
Ayers PW (2008) The continuity of the energy and other molecular properties with respect to the number of electrons. J Math Chem 43(1):285–303
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44(2):129–138
Valone SM (2011) Quantum mechanical origins of the Iczkowski–Margrave model of chemical potential. J Chem Theory Comp 7(7):2253–2261
Cohen MH, Wasserman A (2007) On the foundations of chemical reactivity theory. J Chem Phys A 111(11):2229–2242
Ayers PW (2007) On the electronegativity nonlocality paradox. Theor Chem Acc 118(2):371–381
Coleman AJ, Yukalov VI (2000) Reduced density matrices: Coulson’s challenge. Springer, New York
Gatti C, Macchi P (eds) (2012) Modern charge-density analysis. Springer, Dordrecht
Fradera X, Austen MA, Bader RFW (1999) The Lewis model and beyond. J Phys Chem A 103(2):304–314
Alcoba DR, Torre A, Lain L, Bochicchio RC (2005) Energy decompositions according to physical space partitioning schemes. J Chem Phys 122(7):074102
Alcoba DR, Lain L, Torre A, Bochicchio RC (2005) A study of the partitioning of the first-order reduced density matrix according to the theory of atoms in molecules. J Chem Phys 123(14):144113
Becke AD (1988) A multicenter numerical integration scheme for polyatomic molecules. J Chem Phys 88(4):2547–2553
Torre A, Alcoba DR, Lain L, Bochicchio RC (2005) Determination of three-center bond indices from population analysis: a fuzzy atom treatment. J Phys Chem A 109(29):6587–6591
Ter Haar D (1961) Theory and applications of the density matrix. Rep Prog Phys 24(1):304–362
Blum K (1981) Density matrix theory and applications. Plenum, New York
Emch GG (1972) Algebraic methods in statistical mechanics and quantum field theory. Wiley-Interscience, New York
Blaizot JP, Ripka G (2006) Quantum theory of finite systems. The MIT Press, Cambridge
Rosina M (2001) In: Cioslowsky J (ed) Many-electron densities and reduced density matrices. Kluwer, Dordrecht
Mazziotti DA (1998) Contracted Schrödinger equation: determining quantum energies and two-particle density matrices without wave functions. Phys Rev A 57(6):4219–4234
Hellgren M, Gross EKU (2012) Effect of discontinuities in Kohn–Sham-based chemical reactivity theory. J Chem Phys 136(11):114102
McWeeny R (2001) Methods of molecular quantum mechanics. Academic, San Diego
Kummer H (1967) n-Representability problem for reduced density matrices. J Math Phys 8(10):2063–2081
Paldus J (1976) In: Eyring H, Henderson DJ (eds) Theoretical chemistry: advances and perspectives, vol 2. Academic Press, New York
Surján PR (1989) Second quantized approach to quantum chemistry an elementary introduction. Springer-Verlag, Berlin
Mazziotti DA (ed) (2007) Reduced-Density-matrix mechanics: with application to many-electron atoms and molecules. Advances in chemical physics 134. Wiley
Valdemoro C (1996) Contracting and calculating traces over the N-electron space: two powerful tools for obtaining averages. Int J Quant Chem 60(1):131–139
Miranda-Quintana RA, Bochicchio RC (2014) Energy dependence with the number of particles: density and reduced density matrices functionals. Chem Phys Lett 593(1):35–39
Parr RG, Yang WT (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106(14):4049–4050
Yang WT, Parr RG, Pucci R (1984) Electron density, Kohn–Sham frontier orbitals, and Fukui functions. J Chem Phys 81(6):2862–2863
Ayers P, Levy M (2000) Perspective on “Density functional approach to the frontier-electron theory of chemical reactivity”. Theor Chem Acc 103(3–4):353–360
Bultinck P, Cardenas C, Fuentealba P, Johnson PA, Ayers PW (2014) How to compute the Fukui matrix and function for systems with (quasi-)degenerate states. J Chem Theory Comp 10(1):202–210
Bultinck P, Clarisse D, Ayers PW, Carbo-Dorca R (2011) The Fukui matrix: a simple approach to the analysis of the Fukui function and its positive character. Phys Chem Chem Phys 13(13):6110–6115
Cioslowski J, Stefanov BB (1993) Electron flow and electronegativity equalization in the process of bond formation. J Chem Phys 99(7):5151–5162
Parker SM, Shiozaki T (2014) Active space decomposition with multiple sites: density matrix renormalization group algorithm. J Chem Phys 141(21):211102 and references
Tapia O, Bertrán J (eds) (2003) Solvent effects in chemical reactivity. Kluwer, New York
Surján PR, Ángyán J (1983) Perturbation theory for nonlinear time-independent Schrödinger equations. Phys Rev A 28(1):45–48
Mead CA, Truhlar DG (1982) Conditions for the definition of a strictly diabatic electronic basis for molecular systems. J Chem Phys 77(12):6090–6098
Davies EB (1976) Quantum theory of open systems. Academic Press, London
Attal S, Joye A, Pillet CA (eds) (2006) Open quantum systems I. The Hamiltonian approach. In: Lecture Notes in Mathematics. Springer, Berlin
Cohen-Tannoudji C, Diu B, Laloë F (1991) Quantum mechanics, vol 1. Wiley, New York
Acknowledgments
This report has been financially supported by Projects 20020130100226BA (Universidad de Buenos Aires) and PIP No. 11220090100061 (Consejo Nacional de Investigaciones Científicas y Técnicas, República Argentina).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “Festschrift in honour of P. R. Surjan”.
Rights and permissions
About this article
Cite this article
Bochicchio, R.C. On the non-integer number of particles in molecular system domains: treatment and description. Theor Chem Acc 134, 138 (2015). https://doi.org/10.1007/s00214-015-1743-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-015-1743-2