Abstract
Rationale
Recently, it has been suggested that isoflurane might reduce dopamine release from rat midbrain dopaminergic neurons, the neurobiological substrate implicated in the reinforcing effects of abused drugs and nondrug rewards. However, little is known about effects of isoflurane on neurobehavioral activity associated with chronic exposure to psychoactive substances.
Objective
The present study was designed to investigate the effects of isoflurane on cocaine-reinforced behavior. Using behavioral paradigm in rats, we evaluated the effects of isoflurane on cocaine self-administration under fixed ratio (FR) and progressive ratio (PR) schedules of reinforcement. We also tested the effects of isoflurane on lever responding by nondrug reinforcers (sucrose and food) in drug-naive rats to control for the nonselective effects of isoflurane on cocaine- and nicotine-taking behavior. To further assess the ability of isoflurane to modulate the motivation for taking a drug, we evaluated the effects of isoflurane on nicotine self-administration. Using different groups of rats, the effects of isoflurane on the locomotor activity induced by a single intraperitoneal injection of cocaine (15 mg/kg) were also examined.
Results
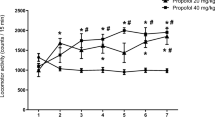
Isoflurane significantly suppressed the self-administration of cocaine and nicotine without affecting food consumption. Unlike food-reinforced responding, responding for sucrose reinforcement was decreased by isoflurane. Isoflurane reduced breaking points under a PR schedule of reinforcement in a dose-dependent manner, indicating its efficacy in decreasing the incentive value of cocaine. Isoflurane also attenuated acute cocaine-induced hyperlocomotion.
Conclusions
The results provided evidence that isoflurane decreases cocaine- and nicotine-reinforced responses, while isoflurane effect is not selective for cocaine- and nicotine-maintained responding. These results suggest that isoflurane inhibitions of cocaine- and nicotine-maintenance responses may be related to decreased effects of dopamine, and further investigation will need to elucidate this relationship.







Similar content being viewed by others
References
Adachi YU, Aramaki Y, Satomoto M, Higuchi H, Watanabe K (2003) Halothane attenuated haloperidol and enhanced clozapine-induced dopamine release in the rat striatum. Neurochem Int 43:113–119
Adachi YU, Yamada S, Satomoto M, Higuchi H, Watanabe K, Kazama T, Mimuro S, Sato S (2008) Isoflurane anesthesia inhibits clozapine- and risperidone-induced dopamine release and anesthesia-induced changes in dopamine metabolism was modified by fluoxetine in the rat striatum: an in vivo microdialysis study. Neurochem Int 52:384–391
Antinori S, Fattore L, Saba P, Fratta W, Gessa GL, Devoto P (2018) Levodopa prevents the reinstatement of cocaine self-administration in rats via potentiation of dopamine release in the medial prefrontal cortex. Addict Biol 23:556–568
Bachtell RK, Whisler K, Karanian D, Self DW (2005) Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology 183:41–53
Balster RL (1998) Neural basis of inhalant abuse. Drug Alcohol Depend 51:207–214
Banks MI, Pearce RA (1999) Dual actions of volatile anesthetics on GABA(A) IPSCs: dissociation of blocking and prolonging effects. Anesthesiology 90:120–134
Bari AA, Pierce RC (2005) D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience 135:959–968
Beninger RJ (1983) The role of dopamine in locomotor activity and learning. Brain Res 287:173–196
Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A (2006) Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49:589–601
Caine SB, Koob GF (1994) Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 270:209–218
Caine SB, Negus SS, Mello NK, Bergman J (1999) Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther 291:353–360
Corrigall WA, Coen KM (1991) Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology 104:171–176
Corrigall WA, Franklin KB, Coen KM, Clarke PB (1992) The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology 107:285–289
DiPalma D, Rezvani AH, Willette B, Wells C, Slade S, Hall BJ, Levin ED (2019) Persistent attenuation of nicotine self-administration in rats by co-administration of chronic nicotine infusion with the dopamine D1 receptor antagonist SCH-23390 or the serotonin 5-HT2C agonist lorcaserin. Pharmacol Biochem Behav 176:16–22
Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW (2007) Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology 32:354–366
el-Maghrabi EA, Eckenhoff RG (1993) Inhibition of dopamine transport in rat brain synaptosomes by volatile anesthetics. Anesthesiology 78:750–756
Ferraro TN, Golden GT, Berrettini WH, Gottheil E, Yang CH, Cuppels GR, Vogel WH (2000) Cocaine intake by rats correlates with cocaine-induced dopamine changes in the nucleus accumbens shell. Pharmacol Biochem Behav 66:397–401
Garcia PS, Kolesky SE, Jenkins A (2010) General anesthetic actions on GABA(A) receptors. Curr Neuropharmacol 8:2–9
Gardner EL, Schiffer WK, Horan BA, Highfield D, Dewey SL, Brodie JD, Ashby CR Jr. (2002) Gamma-vinyl GABA, an irreversible inhibitor of GABA transaminase, alters the acquisition and expression of cocaine-induced sensitization in male rats. Synapse 46:240–250
** W, Kim MS, Jang EY, Lee JY, Lee JG, Kim HY, Yoon SS, Lee BH, Chang S, Kim JH, Choi KH, Koo H, Gwak YS, Steffensen SC, Ryu YH, Kim HY, Yang CH (2018) Acupuncture reduces relapse to cocaine-seeking behavior via activation of GABA neurons in the ventral tegmental area. Addict Biol 23:165–181
Keita H, Henzel-Rouelle D, Dupont H, Desmonts JM, Mantz J (1999) Halothane and isoflurane increase spontaneous but reduce the N-methyl-D-aspartate-evoked dopamine release in rat striatal slices: evidence for direct presynaptic effects. Anesthesiology 91:1788–1797
Kohut SJ, Hiranita T, Hong SK, Ebbs AL, Tronci V, Green J, Garces-Ramirez L, Chun LE, Mereu M, Newman AH, Katz JL, Tanda G (2014) Preference for distinct functional conformations of the dopamine transporter alters the relationship between subjective effects of cocaine and stimulation of mesolimbic dopamine. Biol Psychiatry 76:802–809
Koob GF (2021) Drug Addiction: Hyperkatifeia/Negative Reinforcement as a framework for medications development. Pharmacol Rev 73:163–201
Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP (2004) Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27:739–749
Kotani N, Akaike N (2013) The effects of volatile anesthetics on synaptic and extrasynaptic GABA-induced neurotransmission. Brain Res Bull 93:69–79
Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G (2007) Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology 191:653–667
Lecca D, Cacciapaglia F, Valentini V, Gronli J, Spiga S, Di Chiara G (2006) Preferential increase of extracellular dopamine in the rat nucleus accumbens shell as compared to that in the core during acquisition and maintenance of intravenous nicotine self-administration. Psychopharmacology 184:435–446
Liu QS, Pu L, Poo MM (2005) Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature 437:1027–1031
Mathon DS, Lesscher HM, Gerrits MA, Kamal A, Pintar JE, Schuller AG, Spruijt BM, Burbach JP, Smidt MP, van Ree JM, Ramakers GM (2005) Increased gabaergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice. Neuroscience 130:359–367
Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT (2014) Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 82:1346–1356
McDougall SA, Rios JW, Apodaca MG, Park GI, Montejano NR, Taylor JA, Moran AE, Robinson JAM, Baum TJ, Teran A, Crawford CA (2020) Effects of dopamine and serotonin synthesis inhibitors on the ketamine-, d-amphetamine-, and cocaine-induced locomotor activity of preweanling and adolescent rats: sex differences. Behav Brain Res 379:112302
Medvedev IO, Ramsey AJ, Masoud ST, Bermejo MK, Urs N, Sotnikova TD, Beaulieu JM, Gainetdinov RR, Salahpour A (2013) D1 dopamine receptor coupling to PLCbeta regulates forward locomotion in mice. J Neurosci 33:18125–18133
Michel FJ, Trudeau LE (2000) Clozapine inhibits synaptic transmission at GABAergic synapses established by ventral tegmental area neurons in culture. Neuropharmacology 39:1536–1543
Nakamura Y, Longueville S, Nishi A, Herve D, Girault JA, Nakamura Y (2021) Dopamine D1 receptor-expressing neurons activity is essential for locomotor and sensitizing effects of a single injection of cocaine. Eur J Neurosci.
Nugent FS, Kauer JA (2008) LTP of GABAergic synapses in the ventral tegmental area and beyond. J Physiol 586:1487–1493
Olmstead MC (2011) Animal models of drug addiction. Humana Press, New York
Pan B, Hillard CJ, Liu QS (2008) D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci 28:14018–14030
Pascoli V, Terrier J, Hiver A, Luscher C (2015) Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 88:1054–1066
Pierce RC, Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30:215–238
Pulvirenti L, Koob GF (1994) Dopamine receptor agonists, partial agonists and psychostimulant addiction. Trends Pharmacol Sci 15:374–379
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Rodefer JS, Campbell UC, Cosgrove KP, Carroll ME (1999) Naltrexone pretreatment decreases the reinforcing effectiveness of ethanol and saccharin but not PCP or food under concurrent progressive-ratio schedules in rhesus monkeys. Psychopharmacology 141:436–446
Romach MK, Schoedel KA, Sellers EM (2014) Human abuse liability evaluation of CNS stimulant drugs. Neuropharmacology 87:81–90
Schindler CW, Carmona GN (2002) Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Behav 72:857–863
Siegal N, Dow-Edwards D (2009) Isoflurane anesthesia interferes with the expression of cocaine-induced sensitization in female rats. Neurosci Lett 464:52–56
Topf N, Jenkins A, Baron N, Harrison NL (2003) Effects of isoflurane on gamma-aminobutyric acid type A receptors activated by full and partial agonists. Anesthesiology 98:306–311
Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324:1080–1084
Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N, Nakanishi S (1999) Isoflurane anesthesia enhances the inhibitory effects of cocaine and GBR12909 on dopamine transporter: PET studies in combination with microdialysis in the monkey brain. Brain Res 849:85–96
van Zessen R, Phillips JL, Budygin EA, Stuber GD (2012) Activation of VTA GABA neurons disrupts reward consumption. Neuron 73:1184–1194
Velasquez-Martinez MC, Santos-Vera B, Velez-Hernandez ME, Vazquez-Torres R, Jimenez-Rivera CA (2020) Alpha-1 adrenergic receptors modulate glutamate and gaba neurotransmission onto ventral tegmental dopamine neurons during cocaine sensitization. Int J Mol Sci 21.
Vezina P (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev 27:827–839
Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F (2011) Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 108:15037–15042
Watanabe-Uchida M, Narukawa M (2017) Utilization of population pharmacokinetics in drug development and provision of the results to healthcare professionals. Int J Clin Pharmacol Ther 55:25–31
Westphalen RI, Hemmings HC Jr. (2006) Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: basal release. J Pharmacol Exp Ther 316:208–215
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
** ZX, Stein EA (1998) Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain Res 798:156–165
** ZX, Stein EA (2002) GABAergic mechanisms of opiate reinforcement. Alcohol Alcohol 37:485–494
Yang H, de Jong JW, Tak Y, Peck J, Bateup HS, Lammel S (2018) Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron 97(434–449):e4
Yoon SS, Kim H, Choi KH, Lee BH, Lee YK, Lim SC, Choi SH, Hwang M, Kim KJ, Yang CH (2010) Acupuncture suppresses morphine self-administration through the GABA receptors. Brain Res Bull 81:625–630
Zimmerman SA, Jones MV, Harrison NL (1994) Potentiation of gamma-aminobutyric acidA receptor Cl- current correlates with in vivo anesthetic potency. J Pharmacol Exp Ther 270:987–991
Funding
This research was supported by a grant (20182MFDS422 and 20182MFDS425) from the Ministry of Food and Drug Safety in 2021 and National Research Foundation of Korea (NRF) grant funded by the Korea government MSIT (No. 2018R1A5A2025272, No.2019R1I1A1A01052581, No. 2020R1A2C1006559).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoon, S.S., Lee, B.H., Lee, S.H. et al. Effects of isoflurane anesthesia on addictive behaviors in rats. Psychopharmacology 239, 3621–3632 (2022). https://doi.org/10.1007/s00213-022-06236-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06236-z