Abstract
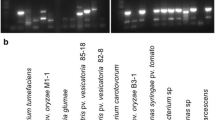
Among biotic stresses, Alternaria leaf spots caused by Alternaria brassicae and A. brassicicola and black rot caused by Xanthomonas campestris pv. campestris are major limiting factors in brassica cultivation across the world. Because of seed-borne nature of these pathogens primarily, disease-free conservation as well as exchange of brassica seeds at domestic as well as international level are major challenges. To facilitate disease-free conservation and transboundary movement of brassica germplasm, a highly specific and sensitive method was developed for simultaneous detection of these pathogens. A set of primers namely, AbeABC1F and AbeABC1R based on ABC transporter (Atr1) gene for A. brassicae, Aba28sF and Aba28sR based on SSR marker was developed for A. brassicicola as well as rpf gene-based primers namely, rpfH_F and rpfH_R for X. campestris pv. campestris were used for multiplex PCR. The specific bands of 586, 201 and 304 bp were obtained in multiplex PCR assay for A. brassicae, A. brassicicola and X. campestris pv. campestris, respectively. Therefore, the developed multiplex PCR protocol could be utilized for a reliable diagnosis of these pathogens to facilitate safe conservation, exchange of seeds to the researchers and also by seed certification agencies for ensuring quality seed availability to farmers.




Similar content being viewed by others
Code availability
Not applicable.
References
Akhtar J, Kandan A, Singh B, Dev U, Chand D, Kumar J, Agarwal PC (2014) Modified isolation technique for obtaining pure cultures of seedborne fungi. Indin J Plant Prot 42:156–159
Akhtar J, Singh B, Kandan A, Kumar P, Maurya AK, Chand D (2017a) Survival of Alternaria brassicicola in cryo-preserved Brassica spp. seeds. Indian Phytopathol 70(2):256–257
Akhtar J, Singh B, Kandan A, Kumar P, Maurya AK, Dubey SC (2017b) Interception of pathogens during quarantine processing: an effort towards safe import of oilseed and vegetable Brassicas germplasm in India. J Oilseed Brassica 81(2):120–130
Baek KY, Lee HH, Son GJ, Lee PA, Roy N, Seo YS, Lee SW (2018) Specific and sensitive primers developed by comparative genomics to detect bacterial pathogens in grains. Plant Pathol J 34(2):104–112
Chakdar H, Goswami SK, Singh E, Choudhary P, Yadav J, Kashyap PL, Srivastava AK, Saxena AK (2019) noxB-based marker for Alternaria spp.: a new diagnostic marker for specific and early detection in crop plants. Biotech 9(7):249
Guillemette T, Iacomi-Vasilescu B, Simoneau P (2004) Conventional and real-time PCR-based assay for detecting pathogenic Alternaria brassicae in cruciferous seed. Plant Dis 88(5):490–496
Humpherson-Jones FM, Phelps K (1989) Climatic factors influencing spore production in Alternaria brassicae and Alternaria brassicicola. Ann Appl Biol 114(3):449–458
Iacomi-Vasilescu B, Blanchard D, Guenard M, Molinero-Demilly V, Laurent E, Simoneau P (2002) Development of a PCR based diagnostic assay for detecting pathogenic Alternaria species in cruciferous seeds. Seed Sci Technol 30(1):87–96
Ignatov A, Sechler A, Schuenzel EL, Agarkova I, Oliver B, Vidaver AK, Schaad NW (2007) Genetic diversity in populations of Xanthomonas campestris pv. campestris in cruciferous weeds in central coastal California. Phytopathology 97(7):803–812
Inoue Y, Fujikawa T, Takikawa Y (2021) Detection and identification of Xanthomonas campestris pv. campestris and pv. raphani by multiplex polymerase chain reaction using specific primers. Appl Microbiol Biotechnol 105:1991–2002
Kang IJ, Kang MH, Noh TH, Shim HK, Shin DB, Heu S (2016) Simultaneous detection of three bacterial seed-borne diseases in rice using multiplex polymerase chain reaction. Plant Pathol J 32(6):575–579
Kiran R, Kandan A, Kumar P, Singh D, Akhtar J, Singh B, Dubey SC (2019) Development of species-specific primers for detection of Xanthomonas campestris pv. campestris causing black rot of crucifers. J Environ Biol 40(1):105–110
Kumar P, Kiran R, Kandan A, Akhtar J, Singh B, Nair K, Dubey SC (2017) Detection of Xanthomonas campestris pv campestris in imported germplasm of cabbage during post entry quarantine. Indian Phytopathol 70(4):413–417
Kumar P, Akhtar J, Kandan A, Singh B, Kiran R, Nair K, Dubey SC (2018) Efficacy of URP and ISSR markers to determine diversity of indigenous and exotic isolates of Curvularia lunata. Indian Phytopathol 71:235–242
Kumar P, Dubey SC, Akhtar J, Kiran R, Nair K, Bhati H (2020) Genetic diversity and population structure analysis of Colletotrichum capsici isolates using SRAP and URP markers. Indian J Plant Prot 48:64–73
Lee C, Lee HH, Mannaa M, Kim N, Park J, Kim J, Seo YS (2018) Genomics-based sensitive and specific novel primers for simultaneous detection of Burkholderia glumae and Burkholderia gladioli in rice seeds. Plant Pathol J 34(6):490–498
Leu YS, Deng WL, Yang WS, Wu YF, Cheng AS, Hsu S, Tzeng KC (2010) Multiplex polymerase chain reaction for simultaneous detection of Xanthomonas campestris pv. campestris and X. campestris pv. raphani. Plant Pathol Bull 19:137–147
Mathur SB, Kongsdal O (2003) Common laboratory seed health testing methods for detecting fungi. International Seed Testing Association, Bassersdorf, Switzerland
Meena PD, Chattopadhyay C, Meena SS, Kumar A (2011) Area under disease progress curve and apparent infection rate of Alternaria blight disease of Indian mustard (Brassica juncea) at different plant age. Arch Phytopathol Pflanzenschutz 44(7):684–693
Meena M, Gupta SK, Swapnil P, Zehra A, Dubey MK, Upadhyay RS (2017) Alternaria toxins: Potential virulence factors and genes related to pathogenesis. Front Microbiol 8:1451
Potnis N, Krasileva K, Chow V, Almeida NF, Patil PB, Ryan RP et al (2011) Comparative genomics reveals diversity among xanthomonads infecting tomato and pepper. BMC Genom 12(1):146
Prasad L, Vishunavat K (2006) Assessment of yield loss in cauliflower seed crop due to Alternaria blight. Indian Phytopathol 59(2):185–189
Ram RS, Chauhan VB (1998) Assessment of yield losses due to Alternaria leaf spot in various cultivars of mustard and rapeseed. J Mycopathol Res 36(2):109–111
Randhawa PS, Schaad NW (1984) Selective isolation of Xanthomonas campestris pv. campestris from crucifer seeds. Phytopathology 74(3):268–272
Schaad NW, Alvarez A (1993) Xanthomonas campestris pv. campestris: cause of black rot of crucifers. In: Swings JG, Civerolo EL (eds) Xanthomonas. Chapman & Hall, London, pp 51–55
Schaad NW, Sitterly WR, Humaydan H (1980) Relationship of incidence of seedborne Xanthomonas campestris to black rot of crucifers. Plant Dis 64(1):91–92
Sharma P, Deep S, Sharma M, Bhati DS (2013) Genetic variation of Alternaria brassicae (Berk) Sacc, causal agent of dark leaf spot of cauliflower and mustard in India. J Gen Plant Pathol 79(1):41–45
Shrestha K, Shrestha SK (1992) Major diseases of rapeseed-mustard and their control measures. In: Proceedings of the First HMG/DANIDA/FAO National training course of seed production of rapeseed-mustard crops, pp 131–160
Singh R, Kumar S, Kashyap PL, Srivastava AK, Mishra S, Sharma AK (2014) Identification and characterization of microsatellite from Alternaria brassicicola to assess cross-species transferability and utility as a diagnostic marker. Mol Biotechnol 56(11):1049–1059
Singh B, Akhtar J, Kandan A, Kumar P, Chand D, Maurya AK, Agarwal PC, Dubey SC (2018) Risk of pathogens associated with plant germplasm imported into India from various countries. Indian Phytopathol 71(1):91–102
Tripathi A, Rai A, Dubey SC, Akhtar J, Kumar P (2021) DNA barcode, multiplex PCR and qPCR assay for diagnosis of pathogens infecting pulse crops to facilitate safe exchange and healthy conservation of germplasm. Arch Microbiol 203:2575–2589
Vicente JG, Holub EB (2013) Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol Plant Pathol 14:2–18
Williams PH (1980) Black rot: a continuing threat to world crucifers. Plant Dis 64(8):736–742
Wilson K (2001) Preparation of genomic DNA from bacteria. Curr Protocol Mol Biol 56(1):2–4
Acknowledgements
Authors gratefully acknowledge the Director, ICAR-NBPGR for support and encouragement, ITCC, ICAR-IARI, New Delhi, NAIMCC, ICAR-NBAIM, Mau for providing cultures and Indian Council of Agricultural Research, New Delhi, India for financial support.
Funding
This study was financially supported by Indian Council of Agricultural Research (ICAR), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics approval
The research does not involve any human participation and/or animals. The materials in the article have not been published in whole or in part elsewhere and not currently being considered for publication in another journal.
Consent to participate
Yes.
Consent for publication
Yes.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiran, R., Kumar, P., Akhtar, J. et al. Development of multiplex PCR assay for detection of Alternaria brassicae, A. brassicicola and Xanthomonas campestris pv. campestris in crucifers. Arch Microbiol 204, 224 (2022). https://doi.org/10.1007/s00203-022-02846-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02846-5