Abstract
Introduction and hypothesis
To investigate the tissue reactions of a novel porcine-derived urinary bladder matrix/small intestinal submucosa (UBM/SIS) biological mesh and SIS mesh implanted in a rabbit vaginal defect model.
Methods
Thirty-two rabbits were implanted with UBM/SIS mesh (Group A) and SIS mesh (Group B), respectively. Rabbits were sacrificed at 7, 14, 60, and 180 days after implantation. The tensile strength, elongation at break, and elastic modulus of the tissue were measured using biomechanical methods. The inflammatory response, cell infiltration, vascularization, and collagen fibers were observed.
Results
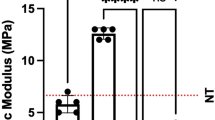
Compared with Group B, the tensile strength and elongation at break of group A was higher at 14, 60, and 180 days. The elastic modulus of group A was lower at 180 days. Inflammatory response of group A was milder at 14, 60, and 180 days. There was more cell infiltration in group A at 7 and 14 days. Vascularization was higher in group A at 7 days and 14 days. The order of collagen in group A was better at 14, 60, and 180 days. The proportion of thick red fibers in both groups showed an increasing trend. At 14 days, group A had more thick red fibers.
Conclusions
The novel UBM/SIS composite mesh had a milder inflammatory response; earlier induction of cell infiltration, angiogenesis, and collagen regeneration. Collagen fibers had a better order. It has a higher tensile strength and greater elongation at break, and can be used as a potential material for the treatment of pelvic organ prolapse.








Similar content being viewed by others
References
Buchsbaum GM, Lee TG, et al. Vaginal obliterative procedures for pelvic organ prolapse: a systematic review. Obstet Gynecol Surv. 2017;72(3):175–83.
Yang J, Han J, Zhang K, et al. Outcomes of implanting porcine small intestinal submucosa mesh in rabbit vesicovaginal space. Chin J Obstet Gynecol. 2020;55(2):120–4.
Jayadev R, Sherwood DR, et al. Basement membranes. Curr Biol: CB. 2017;27(6):R207–11.
Fan X, Wang Y, et al. Comparison of polypropylene mesh and porcine-derived, cross-linked urinary bladder matrix materials implanted in the rabbit vagina and abdomen. Int Urogynecol J. 2014;25(5):683–9.
Bauman TM, Nicholson TM, Abler LL, et al. Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS One. 2014;9(10):e109102.
Sadtler K, Sommerfeld SD, et al. Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury. Semin Immunol. 2017;29:14–23.
Yao Y, Zhang K, Han J, et al. Tissue reactions to heterogenic and allogeneic acellular dermal matrix mesh placed in the vesicovaginal space in a rabbit model. Gynecol Obstet Invest. 2017;82(5):437–55.
Hussey GS, Dziki JL, Badylak SF. Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater. 2018;3:159–73.
Brown B, Lindberg K, Reing J, et al. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006b;12(3):519–26.
Zhang H, Zhang Q, et al. Urinary bladder matrix scaffolds improve endometrial regeneration in a rat model of intrauterine adhesions. Biomater Sci. 2020;8(3):988–96.
Stehbens WE, Silver MD. Unusual development of basement membrane about small blood vessels. J Cell Biol. 1965;6(2):669–72.
Lindberg K, Badylak SF (2001) Porcine small intestinal submucosa (SIS): a bioscaffold supporting in vitro primary human epidermal cell differentiation and synthesis of basement membrane proteins. Burns. 2001;27(3):254-266.
Carbonetto S. The extracellular matrix of the nervous system. TINS. 1984;7:382–7.
Lynn Y, Goon TH, et al. Laminins in cellular differentiation. Trends Cell Biol. 2019;29(12):987–1000.
Perry G, **ao W, et al. Engineered basement membranes: from in vivo considerations to cell-based assays. Integr Biol. 2018;10:680–95.
Tocce EJ, Gasiorowski JZ, et al. Engineering the biophysical properties of basement membranes into biomaterials: fabrication and effects on cell behavior. Comprehensive Biomater. 2017;II(4):404–29.
Tyler Miller R. Mechanical properties of basement membrane in health and disease. Matrix Biol. 2017;57–58:366–73.
Abrams GA, Murphy CJ. Ultrastructural basement membrane topography of the bladder epithelium. Urol Res. 2003;31:341–6.
Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22(2):97–104.
Sharf Y, Knubovets T, Dayan D, et al. The source of the NMR detected motional anisotropy of water in blood vessel walls. Biophys J. 1997;73:1198–204.
Brown BN, Ratner BD, et al. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33(15):3792–802.
Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15(7):602–11.
Chen M, Marinkovich MP, et al. Interactions of the amino-terminal noncollagenous (NC1) domain of Type VII collagen with extracellular matrix components. A potential role in epidermal–dermal adherence in human skin. Biol Chem. 1997;272(23):14516–22.
Londono R, Stephen F, et al. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43(3):577–92.
Rousselle P, Montmasson M, Garnier C. Extracellular matrix contribution to skin wounds re-epithelialization. Matrix Biol. 2019;75–76:12–26.
Pierce Lisa M, Grunlan Melissa A, Hou Ya**, et al. Biomechanical properties of synthetic and biologic graft materials following long term implantation in the rabbit abdomen and vagina. AJOG. 2009;200(5):549(e1).
Charvolin J, Sadoc J-F. Type-I collagen fibrils: from growth morphology to local order Eur Phys J E Soft Matter. 2019;42(4):49.
Acknowledgements
The present work was supported by Peking University Clinical Medicine +X Youth Special Project (A 82504-03)
Funding
Peking University Clinical Medicine + X Youth Special Project,A82504-03,Yiting Wang
Author information
Authors and Affiliations
Contributions
Yiting Wang: experiments on animals, data collection, manuscript writing.
Kun Zhang: experiments on animals
Junfang Yang: experiments on animals
Ying Yao: experiments on animals
Yiqi Guan: experiments on animals
Wenyue Cheng: data collection
**song Han: project development
Jian Zhang: project development
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Zhang, K., Yang, J. et al. Outcome of a novel porcine-derived UBM/SIS composite biological mesh in a rabbit vaginal defect model. Int Urogynecol J 34, 1501–1511 (2023). https://doi.org/10.1007/s00192-022-05400-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05400-5