Abstract
Purpose
The purpose of this study was to evaluate acute skin toxicity in early breast cancer patients treated with hypofractionated radiotherapy (HFRT) after breast-conserving surgery and to identify factors predictive for grade ≥ 2 acute skin toxicity.
Materials and methods
A monocentric retrospective study was carried out using cases treated between December 2017 and November 2020. We analyzed data from 202 patients with early breast cancer treated with 3D hypofractionated RT (40.05 Gy in 15 fractions) to the whole breast with or without regional lymph nodes, followed by 13.35 Gy in 5 fractions to the tumor bed. Acute skin toxicity was monitored during RT according to CTCAE (common toxicity criteria for adverse events) scale. Univariate and multivariate analyses were performed to assess predictive factors of acute skin toxicity.
Results
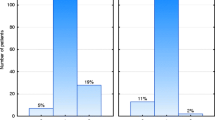
Overall, there was no erythema in 9%, grade 1 erythema in 64.5%, grade 2 in 24%, and grade 3 in 2.5%. No grade 4 erythema was seen. Median delay between RT initiating and maximum skin reaction was 22 days (range 4–44 days). No patient interrupted treatment. In univariate analysis, the rate of acute skin toxicity grade 2--–3 (G2-3) was significantly higher for patients with larger tumor size (p = 0.02), body mass index > 27 (p = 0.04), and time between chemotherapy (CT) and RT less than 20 days (p = 0.01). Dosimetric risk factors for acute skin toxicity G2‑3 were breast volume > 800 cc (p = 0.000), boost volume > 18 cc (p = 0.002), V105% > 40 cc (p = 0.03), and Dmax > 56 Gy (p = 0.007). CT, trastuzumab, regional lymph node radiation, and age were not correlated with increased skin toxicity. In multivariate analysis, acute skin toxicity correlated with T stage (p = 0.032), breast volume > 800 cc (p = 0.012), boost volume > 18 cc (p = 0.04), and Dmax > 56 Gy (p = 0.035).
Conclusion
Our results confirm that whole breast with or without lymph nodes hypofractionated RT is safe and well tolerated. The factors strongly associated with a decreased risk of G2‑3 skin toxicity are T1, breast volume < 800 c, boost volume < 18 cc, and Dmax < 56 Gy. Long-term follow-up is needed to evaluate late toxicity.

Similar content being viewed by others
References
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, Cutter D, Duane F et al (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383(9935):2127–2135
START Trialists’ Group, Bentzen SM, Agrawal RK, Aird EGA, Barrett JM, Barrett-Lee PJ et al (2008) The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 371(9618):1098–1107
Whelan TJ, Kim D‑H, Sussman J (2008) Clinical experience using hypofractionated radiation schedules in breast cancer. Semin Radiat Oncol 18(4):257–264
Shaitelman SF, Schlembach PJ, Arzu I, et al (2015) Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol 1(7):931–941. https://doi.org/10.1001/jamaoncol.2015.2666
Murray Brunt A, Haviland JS, Wheatley DA, et al (2020) Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 395(10237):1613–1626. https://doi.org/10.1016/S0140-6736(20)30932-6
CTEP Common terminology criteria for adverse events (CTCAE) | protocol development. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Accessed 12.2021
Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A et al (2015) ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 114(1):3–10
Sanz J, Rodríguez N, Foro P, et al (2017) Hypofractionated boost after whole breast irradiation in breast carcinoma: chronic toxicity results and cosmesis. Clin Transl Oncol 19(4):464–469. https://doi.org/10.1007/s12094-016-1548-3
Tortorelli G, Di Murro L, Barbarino R, Cicchetti S, di Cristino D, Falco MD et al (2013) Standard or hypofractionated radiotherapy in the postoperative treatment of breast cancer: a retrospective analysis of acute skin toxicity and dose inhomogeneities. Bmc Cancer 13(1):230. https://doi.org/10.1186/1471-2407-13-230
Brunt AM, Wheatley D, Yarnold J, Somaiah N, Kelly S, Harnett A et al (2016) Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3‑week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol 120(1):114–118
Hickey BE, James ML, Lehman M, Hider PN, Jeffery M, Francis DP et al (2016) Fraction size in radiation therapy for breast conservation in early breast cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003860.pub4
Nozaki M, Kagami Y, Shibata T, Nakamura K, Ito Y, Nishimura Y et al (2019) A primary analysis of a multicenter, prospective, single-arm, confirmatory trial of hypofractionated whole breast irradiation after breast-conserving surgery in Japan: JCOG0906. Jpn J Clin Oncol 49(1):57–62. https://doi.org/10.1093/jjco/hyy160
Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J et al (2005) Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol 75(1):9–17
Holloway CL, Panet-Raymond V, Olivotto I (2010) Hypofractionation should be the new ‘standard’ for radiation therapy after breast conserving surgery. Breast 19(3):163–167
Dorn PL, Corbin KS, Al-Hallaq H, Hasan Y, Chmura SJ (2012) Feasibility and acute toxicity of Hypofractionated radiation in large-breasted patients. Int J Radiat Oncol Biol Phys 83(1):79–83
Ciammella P, Podgornii A, Galeandro M, Micera R, Ramundo D, Palmieri T et al (2014) Toxicity and cosmetic outcome of hypofractionated whole-breast radiotherapy: predictive clinical and dosimetric factors. Radiat Oncol 9(1):97. https://doi.org/10.1186/1748-717X-9-97
Harsolia A, Kestin L, Grills I, Wallace M, Jolly S, Jones C et al (2007) Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys 68(5):1375–1380
Hijal T, Al Hamad AA, Niazi T, Sultanem K, Bahoric B, Vuong T et al (2010) Hypofractionated radiotherapy and adjuvant chemotherapy do not increase radiation-induced dermatitis in breast cancer patients. Curr Oncol 17(5):22–27
Kouloulias V, Zygogianni A, Kypraiou E, Georgakopoulos J, Thrapsanioti Z, Beli I et al (2014) Adjuvant chemotherapy and acute toxicity in hypofractionated radiotherapy for early breast cancer. WJCC 2(11):705–710
Zygogianni A, Kouloulias V, Antypas C, Armpilia C, Kyrgias G, Kouvaris J (2014) The impact of intermediate time between chemotherapy and hypofractionated radiotherapy to the radiation induced skin toxicity for breast adjuvant treatment. Breast J 20(1):74–78. https://doi.org/10.1111/tbj.12206
De Langhe S, Mulliez T, Veldeman L, Remouchamps V, van Greveling A, Gilsoul M et al (2014) Factors modifying the risk for develo** acute skin toxicity after whole-breast intensity modulated radiotherapy. Bmc Cancer 14(1):711. https://doi.org/10.1186/1471-2407-14-711
Azria D, Belkacemi Y, Romieu G, Gourgou S, Gutowski M, Zaman K et al (2010) Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer (CO-HO-RT): a phase 2 randomised trial. Lancet Oncol 11(3):258–265
Keenan LG, Lavan N, Dunne M, McArdle O (2019) Modifiable risk factors for acute skin toxicity in adjuvant breast radiotherapy: dosimetric analysis and review of the literature. Med Dosim 44(1):51–55
Taher AN, El-Baradie MM, Essa H, Zaki O, Ezzat S (2004) Hypofractionation versus conventional fractionation radiotherapy after conservative treatment of breast cancer: early skin reactions and cosmetic results. J Egypt Natl Canc Inst 16(3):178–187
De Santis MC, Bonfantini F, Di Salvo F, Dispinzieri M, Mantero E, Soncini F et al (2016) Factors influencing acute and late toxicity in the era of adjuvant hypofractionated breast radiotherapy. Breast 29:90–95
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Ben Amor, M. Bohli, Z. Naimi, D. Aissaoui, N. Mejri, J. Yahyaoui, A. Hamdoun, and L. Kochbati certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Ethical standards
The study has been approved by the institution’s ethics committee. Consent to participate: Informed consent was obtained from all individual participants included in the study. Consent to publish: Patients signed informed consent regarding publishing their data.
Additional information
Data Availability
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben Amor, R., Bohli, M., Naimi, Z. et al. Hypofractionated radiotherapy after breast-conserving surgery: Clinical and dosimetric factors predictive of acute skin toxicity. Strahlenther Onkol 199, 48–54 (2023). https://doi.org/10.1007/s00066-022-01985-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-022-01985-4