Abstract
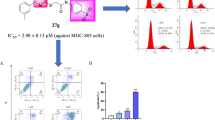
In the current study, a series of pyrimidine derivatives containing the 4-hydroxypiperidine group were designed and synthesized, and the antiproliferative activity of these compounds against four human tumor cell lines (MGC-803, PC-3, A549, H1975) was evaluated by MTT method in vitro. Most of the compounds have moderate anti-proliferative activities, among which compound 17i displayed the most excellent anti-proliferative activity, with IC50 value of 3.89 ± 0.57 µM against H1975 cell. Preliminary antitumor mechanism studies revealed that compound 17i could inhibit colony formation and cell migration of H1975 cells. Furthermore, compound 17i induced H1975 cells apoptosis in a dose-dependent manner and H1975 cell cycle arrest in S phase to inhibit cell proliferation. These results indicates that compound 17i could be a promising lead for further studies.
Graphical Abstract









Similar content being viewed by others
References
Ye T, Han Y, Wang R, Yan P, Chen S, Hou Y, et al. Design, synthesis and biological evaluation of novel 2,4-bismorpholinothieno[3,2-d]pyrimidine and 2-morpholinothieno[3,2-d]pyrimidinone derivatives as potent antitumor agents. Bioorg Chem. 2020;99:103796. https://doi.org/10.1016/j.bioorg.2020.103796
Elmetwally SA, Saied KF, Eissa IH, Elkaeed EB. Design, synthesis and anticancer evaluation of thieno[2,3-d]pyrimidine derivatives as dual EGFR/HER2 inhibitors and apoptosis inducers. Bioorg Chem. 2019;88:102944. https://doi.org/10.1016/j.bioorg.2019.102944
Park JH, Ahn SE, Kim S, Kwon MJ, Suh YJ, Kim D. Complete surgical excision is necessary following vacuum-assisted biopsy for breast cancer. Curr Oncol. 2022;29:9357–64. https://doi.org/10.3390/curroncol29120734
Shanmugalingam A, Hitos K, Hegde S, Al-Mashat A, Pathmanathan N, Edirimmane S, et al. Concordance between core needle biopsy and surgical excision for breast cancer tumor grade and biomarkers. Breast Cancer Res Treat. 2022;193:151–9. https://doi.org/10.1007/s10549-022-06548-w
Kim JK, Marco MR, Roxburgh CSD, Chen CT, Cercek A, Strombom P, et al. Survival after induction chemotherapy and chemoradiation versus chemoradiation and adjuvant chemotherapy for locally advanced rectal cancer. Oncologist. 2022;27:380–8. https://doi.org/10.1093/oncolo/oyac025
Walle T, Kraske JA, Liao B, Lenoir B, Timke C, von Bohlen Und Halbach E, et al. Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8. Sci Adv. 2022;8:eabh4050. https://doi.org/10.1126/sciadv.abh4050
** J, Tang Y, Hu C, Jiang LM, Jiang J, Li N, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J Clin Oncol. 2022;40:1681–92. https://doi.org/10.1200/jco.21.01667.
Zhu X, Cao Y, Liu W, Ju X, Zhao X, Jiang L, et al. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23:e105–e15. https://doi.org/10.1016/s1470-2045(22)00066-3
Dai H, Si X, Wang H, Chi L, Gao C, Wang Z, et al. Design, synthesis and anti-tumor activity evaluation of 4,6,7-substitute quinazoline derivatives. Med Chem Res. 2022;31:1351–68. https://doi.org/10.1007/s00044-022-02897-9
Gomes AR, Pires AS, Abrantes AM, Gonçalves AC, Costa SC, Varela CL, et al. Design, synthesis, and antitumor activity evaluation of steroidal oximes. Bioorg Med Chem. 2021;46:116360. https://doi.org/10.1016/j.bmc.2021.116360
Sun J, Mu J, Wang S, Jia C, Li D, Hua H, et al. Design and synthesis of chromone-nitrogen mustard derivatives and evaluation of anti-breast cancer activity. J Enzyme Inhib Med Chem. 2022;37:431–44. https://doi.org/10.1080/14756366.2021.2018685
Ahmed NM, Youns MM, Soltan MK, Said AM. Design, synthesis, molecular modeling and antitumor evaluation of novel indolyl-pyrimidine derivatives with EGFR inhibitory activity. Molecules. 2021;26. https://doi.org/10.3390/molecules26071838.
Luo G, Ma Y, Liang X, **e G, Luo Y, Zha D, et al. Design, synthesis and antitumor evaluation of novel 5-methylpyrazolo[1,5-a]pyrimidine derivatives as potential c-Met inhibitors. Bioorg Chem. 2020;104:104356. https://doi.org/10.1016/j.bioorg.2020.104356
Finger V, Kufa M, Soukup O, Castagnolo D, Roh J, Korabecny J. Pyrimidine derivatives with antitubercular activity. Eur J Med Chem. 2023;246:114946. https://doi.org/10.1016/j.ejmech.2022.114946
Takasaki I, Watanabe A, Okada T, Kanayama D, Nagashima R, Shudo M, et al. Design and synthesis of pyrido[2,3-d]pyrimidine derivatives for a novel PAC1 receptor antagonist. Eur J Med Chem. 2022;231:114160. https://doi.org/10.1016/j.ejmech.2022.114160
Li G, **ao K, Shi M, Shuai J, Xu Z, Li Z, et al. 4-Oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-d]pyrimidine derivatives: design, synthesis, insecticidal assay and binding mode studies. Chem Biodivers. 2022;19:e202200236. https://doi.org/10.1002/cbdv.202200236
Kang D, Sun Y, Feng D, Gao S, Wang Z, **g L, et al. Development of novel dihydrofuro[3,4-d]pyrimidine derivatives as HIV-1 NNRTIs to overcome the highly resistant mutant strains F227L/V106A and K103N/Y181C. J Med Chem. 2022;65:2458–70. https://doi.org/10.1021/acs.jmedchem.1c01885
Frati L. Reconsidering Otto Warburg’s glycolytic shift: pyrimidine derivatives are effective for the treatment of tumors exerting aerobic glycolysis. Panminerva Med. 2022;64:567–8. https://doi.org/10.23736/s0031-0808.22.04658-4
Ying DX, Wang J, Li XF, Zhang W, Rao GW. Design, synthesis and biological characteristics of pyrazolo[3,4-d]pyrimidine derivatives as potential VEGFR-2 inhibitors. Future Med Chem. 2022;14:1649–62. https://doi.org/10.4155/fmc-2022-0130
Ding R, Wang X, Fu J, Chang Y, Li Y, Liu Y, et al. Design, synthesis and antibacterial activity of novel pleuromutilin derivatives with thieno[2,3-d]pyrimidine substitution. Eur J Med Chem. 2022;237:114398. https://doi.org/10.1016/j.ejmech.2022.114398
Wu R, Liu T, Wu S, Li H, Song R, Song B. Synthesis, antibacterial activity, and action mechanism of novel sulfonamides containing oxyacetal and pyrimidine. J Agric Food Chem. 2022;70:9305–18. https://doi.org/10.1021/acs.jafc.2c02099
Sayed AI, Mansour YE, Ali MA, Aly O, Khoder ZM, Said AM, et al. Novel pyrrolopyrimidine derivatives: design, synthesis, molecular docking, molecular simulations and biological evaluations as antioxidant and anti-inflammatory agents. J Enzyme Inhib Med Chem. 2022;37:1821–37. https://doi.org/10.1080/14756366.2022.2090546
Abdel-Aziz SA, Taher ES, Lan P, Asaad GF, Gomaa HAM, El-Koussi NA, et al. Design, synthesis, and biological evaluation of new pyrimidine-5-carbonitrile derivatives bearing 1,3-thiazole moiety as novel anti-inflammatory EGFR inhibitors with cardiac safety profile. Bioorg Chem. 2021;111:104890. https://doi.org/10.1016/j.bioorg.2021.104890
Denel-Bobrowska M, Olejniczak AB. Non-nucleoside structured compounds with antiviral activity-past 10 years (2010-2020). Eur J Med Chem. 2022;231:114136. https://doi.org/10.1016/j.ejmech.2022.114136
Siniavin AE, Novikov MS, Gushchin VA, Terechov AA, Ivanov IA, Paramonova MP, et al. Antiviral activity of N(1),N(3)-disubstituted uracil derivatives against SARS-CoV-2 variants of concern. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms231710171.
Shelton J, Lu X, Hollenbaugh JA, Cho JH, Amblard F, Schinazi RF. Metabolism, biochemical actions, and chemical synthesis of anticancer nucleosides, nucleotides, and base analogs. Chem Rev. 2016;116:14379–455. https://doi.org/10.1021/acs.chemrev.6b00209
Khan I, Tantray MA, Hamid H, Sarwar Alam M, Sharma K, Kesharwani P. Design, synthesis, in vitro antiproliferative evaluation and GSK-3β kinase inhibition of a new series of pyrimidin-4-one based amide conjugates. Bioorg Chem. 2022;119:105512. https://doi.org/10.1016/j.bioorg.2021.105512
Dolatkhah Z, Javanshir S, Sadr AS, Hosseini J, Sardari S. Synthesis, molecular docking, molecular dynamics studies, and biological evaluation of 4H-chromone-1,2,3,4-tetrahydropyrimidine-5-carboxylate derivatives as potential antileukemic agents. J Chem Inform Model. 2017;57:1246–57. https://doi.org/10.1021/acs.jcim.6b00138
Bantzi M, Augsburger F, Loup J, Berset Y, Vasilakaki S, Myrianthopoulos V, et al. Novel aryl-substituted pyrimidones as inhibitors of 3-mercaptopyruvate sulfurtransferase with antiproliferative efficacy in colon cancer. J Med Chem. 2021;64:6221–40. https://doi.org/10.1021/acs.jmedchem.1c00260
Olszewska B, Stasiak A, McNaught Flores D, Fogel WA, Leurs R, Walczyński K. 4-Hydroxypiperidines and their flexible 3-(Amino)propyloxy analogues as non-imidazole histamine H3 receptor antagonist: further structure−activity relationship exploration and in vitro and in vivo pharmacological evaluation. Int J Mol Sci. 2018;19. https://doi.org/10.3390/ijms19041243.
Choi HS, Rucker PV, Wang Z, Fan Y, Albaugh P, Chopiuk G. et al. R)-2-Phenylpyrrolidine substituted imidazopyridazines: a new class of potent and selective pan-TRK inhibitors. ACS Med Chem Lett. 2015;6:562–7. https://doi.org/10.1021/acsmedchemlett.5b00050.
Magin RS, Liu X, Felix A, Bratt AS, Chan WC, Buhrlage SJ. Small molecules as tools for functional assessment of deubiquitinating enzyme function. Cell Chem Biol. 2021;28:1090–100. https://doi.org/10.1016/j.chembiol.2021.04.021
Turnbull AP, Ioannidis S, Krajewski WW, Pinto-Fernandez A, Heride C, Martin ACL, et al. Molecular basis of USP7 inhibition by selective small-molecule inhibitors. Nature. 2017;550:481–6. https://doi.org/10.1038/nature24451
Barbaraci C, Giurdanella G, Leotta CG, Longo A, Amata E, Dichiara M, et al. Haloperidol metabolite II valproate ester (S)-(-)-MRJF22: preliminary studies as a potential multifunctional agent against uveal melanoma. J Med Chem. 2021;64:13622–32. https://doi.org/10.1021/acs.jmedchem.1c00995
Wang X, Zhang C, Zhang X, Wang J, Zhao L, Zhao D, et al. Design, synthesis and biological evaluation of 2-aminopyrimidine-based LSD1 inhibitors. Bioorg Chem. 2022;121:105699. https://doi.org/10.1016/j.bioorg.2022.105699
Zang Y, Huang L, Chen X, Li C, Ma J, Chen X, et al. Novel nitric oxide-releasing derivatives of pyranocarbazole as antitumor agents: design, synthesis, biological evaluation, and nitric oxide release studies. Eur J Med Chem. 2022;244:114832. https://doi.org/10.1016/j.ejmech.2022.114832
Patel OPS, Arun A, Singh PK, Saini D, Karade SS, Chourasia MK, et al. Pyranocarbazole derivatives as potent anti-cancer agents triggering tubulin polymerization stabilization induced activation of caspase-dependent apoptosis and downregulation of Akt/mTOR in breast cancer cells. Eur J Med Chem. 2019;167:226–44. https://doi.org/10.1016/j.ejmech.2019.02.003
Guo N, Peng Z. MG132, a proteasome inhibitor, induces apoptosis in tumor cells. Asia Pac J Clin Oncol. 2013;9:6–11. https://doi.org/10.1111/j.1743-7563.2012.01535.x
Wei B, Lin Q, Ji YG, Zhao YC, Ding LN, Zhou WJ, et al. Luteolin ameliorates rat myocardial ischaemia-reperfusion injury through activation of peroxiredoxin II. Br J Pharmacol. 2018;175:3315–32. https://doi.org/10.1111/bph.14367
Liu H-J, Zhang X, Gao Y-X, Li J-Z, Wang H-L. Design, synthesis, and antifungal activities of new β-methoxyacrylate analogues. J Chin Chem Soc. 2013;60:27–34. https://doi.org/10.1002/jccs.201200295
O’Dowd CR, Helm MD, Rountree JSS, Flasz JT, Arkoudis E, Miel H, et al. Identification and structure-guided development of pyrimidinone based USP7 inhibitors. ACS Med Chem Lett. 2018;9:238–43. https://doi.org/10.1021/acsmedchemlett.7b00512
Lamberto I, Liu X, Seo HS, Schauer NJ, Iacob RE, Hu W, et al. Structure-guided development of a potent and selective non-covalent active-site inhibitor of USP7. Cell Chem Biol. 2017;24:1490–500.e11. https://doi.org/10.1016/j.chembiol.2017.09.003
Li P, Liu Y, Yang H, Liu HM. Design, synthesis, biological evaluation and structure-activity relationship study of quinazolin-4(3H)-one derivatives as novel USP7 inhibitors. Eur J Med Chem. 2021;216:113291. https://doi.org/10.1016/j.ejmech.2021.113291
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U21A20416).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chi, L., Wang, H., Yu, F. et al. Design, synthesis and antitumor activity evaluation of pyrimidine derivatives containing 4-hydroxypiperidine group. Med Chem Res 32, 2125–2137 (2023). https://doi.org/10.1007/s00044-023-03076-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03076-0