Abstract
Background
Pressure overload-induced pathological cardiac hypertrophy is an independent predecessor of heart failure (HF), which remains the leading cause of worldwide mortality. However, current evidence on the molecular determinants of pathological cardiac hypertrophy is still inadequacy. This study aims to elucidate the role and mechanisms of Poly (ADP-ribose) polymerases 16 (PARP16) in the pathogenesis of pathological cardiac hypertrophy.
Methods
Gain and loss of function approaches were used to demonstrate the effects of genetic overexpression or deletion of PARP16 on cardiomyocyte hypertrophic growth in vitro. Ablation of PARP16 by transducing the myocardium with serotype 9 adeno-associated virus (AAV9)-encoding PARP16 shRNA were then subjected to transverse aortic construction (TAC) to investigate the effect of PARP16 on pathological cardiac hypertrophy in vivo. Co-immunoprecipitation (IP) and western blot assay were used to detect the mechanisms of PARP16 in regulating cardiac hypertrophic development.
Results
PARP16 deficiency rescued cardiac dysfunction and ameliorated TAC-induced cardiac hypertrophy and fibrosis in vivo, as well as phenylephrine (PE)-induced cardiomyocyte hypertrophic responses in vitro. Whereas overexpression of PARP16 exacerbated hypertrophic responses including the augmented cardiomyocyte surface area and upregulation of the fetal gene expressions. Mechanistically, PARP16 interacted with IRE1α and ADP-ribosylated IRE1α and then mediated the hypertrophic responses through activating the IRE1α–sXBP1–GATA4 pathway.
Conclusions
Collectively, our results implicated that PARP16 is a contributor to pathological cardiac hypertrophy at least in part via activating the IRE1α–sXBP1–GATA4 pathway, and may be regarded as a new potential target for exploring effective therapeutic interventions of pathological cardiac hypertrophy and heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac hypertrophy is regarded as an adaptive physiological response of the heart to external stress stimuli, such as pressure overload. However, prolonged cardiac hypertrophy leads to pathological ventricular remodeling and develops into heart failure (HF), which is the final stage of cardiac diseases with high incidence and mortality worldwide [1]. Pathological cardiac hypertrophy is accompanied with enlarged cardiomyocytes area and fetal genes program reactivation, resulting in adverse cardiac remodeling and declined myocardium function [2]. Currently, the combination of angiotensin converting enzyme inhibitors (ACEIs), β-blockers, and aldosterone receptor antagonists are used as the main treatment regimen, however, the overall curative effects are poor. Clearly, new intervention targets are urgently needed to attenuate the progress of pathological cardiac hypertrophy.
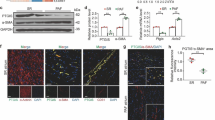
ER stress, caused by the accumulation of misfolded or unfolded proteins, is a common feature accompanied with the cardiovascular diseases progression [3]. The unfolded protein response (UPR), which is elicited by ER stress, activates three transmembrane sensors: Protein Kinase R-like ER Kinase (PERK), Inositol Requiring Enzyme 1α (IRE1α) and the Activating Transcription Factor 6 (ATF6) [4]. Among them, IRE1α is the most highly conserved and could splice 26 nucleotides from the un-spliced XBP1 (uXBP1) mRNA, resulting in frameshift and forming the spliced XBP1 (sXBP1), which functions via a variety of transcriptional targets and participates in numerous cellular stress responses [18]. Therefore, we were curious to determine whether PARP16 regulates GATA4 expression in response to TAC surgery or PE treatment. Here we found that GATA4 was significantly increased in both PE-treated NRCMs and TAC-operated heart tissue while downregulated by PARP16 deficiency (Fig. 8a–c). Similar patterns were observed with the immunofluorescence staining result (Fig. 8d). Furthermore, GATA4 was also activated directly by PARP16 OE, as evidenced by the high protein level and immunofluorescence intensity (Fig. 8e, f). To further confirm the influence of PARP16 on GATA4 localization, nuclear and cytoplasmic proteins were extracted and found PARP16 knockdown markedly decreased the expression of GATA4 in nucleus but not cytoplasm upon PE treatment (Fig. 8g). Collectively, these above results suggested that PARP16 regulates the expression of GATA4 in response to the cardiomyocyte hypertrophic growth.
PARP16 activated GATA4 via IRE1α–sXBP1 pathway in cardiac hypertrophy. a The protein expression of GATA4 in PE-treated NRCMs. b Knockdown of PARP16 abolished the activation of GATA4 in PE-treated NRCMs by western blot assay. c The protein expression of GATA4 was also regulated in heart sections from WT mice injected with AAV9-GFP or AAV9-shPARP16 and subsequently subjected to sham or TAC surgery by immunoblot analysis. d Representative immunofluorescence staining images of GATA4 in PARP16 knockdown followed by PE treatment. scale bar: 50 μm. e PARP16 OE directly activated the expression of GATA4 in NRCMs. f Representative immunofluorescence double staining images of PARP16 and GATA4 in PARP16 OE-transfected H9C2 cells. Scale bar: 50 μm. g The effects of PARP16 knockdown on the nuclear and cytoplasm distribution and expression of GATA4 by western blot. h, i The effects of the ER stress inhibitor (TUDCA) and the specific IRE1α inhibitor STF-083010 on the expressions of GATA4 as well as the hypertrophic markers ANP and BNP in response to PE treatment. j Knockdown of IRE1α abolished the activation of GATA4 or hypertrophic marker BNP in PE-treated NRCMs. k The effects of the specific IRE1α inhibitor STF-083010 on the protein expression of GATA4 as well as the hypertrophic marker ANP and BNP upon PARP16 OE treatment. l The effects of STF-083010 on the mRNA level of GATA4 upon PARP16 OE treatment. Data are presented as mean ± SD and analyzed using Student t test or one-way ANOVA followed by Tukey Post-hoc tests. n = 3. *p < 0.05, **p < 0.01, ***p < 0.001
Interestingly, we have uncovered that PARP16 is an ER transmembrane protein that could interact with IRE1α and activated the IRE1α–sXBP1 pathway during PE-stimulated hypertrophic growth, and GATA4 was a transcription factor primarily located in the nucleus [19], also as evidenced in Fig. 8d, f, g. These outcomes led us to hypothesize that PARP16 may not directly mediate GATA4 activation and maybe through the IRE1α–sXBP1 pathway. To test this hypothesis, both tauroursodeoxycholic acid (TUDCA) (an ER stress inhibitor) and STF-083010 (a specific IRE1α inhibitor) were used to examine the relationship between IRE1α–sXBP1 pathway and GATA4. Indeed, treatment with either TUDCA or STF-083010 could blunt the expressions of GATA4 as well as the hypertrophic marker ANP and BNP in response to PE treatment (Fig. 8h, i). In addition, silencing IRE1α through siRNA upon PE stimulation decreased the protein expressions of GATA4 as well as the hypertrophic marker BNP in NRCMs (Fig. 8j). Importantly, the specific IRE1α inhibitor STF-083010 also attenuated PARP16 OE-induced upregulation of GATA4 both in protein and mRNA levels (Fig. 8k, l), suggesting that PARP16 mediated GATA4 upregulation maybe at least in part via the IRE1α–sXBP1 pathway.
The expression of PARP16 is mediated by JNK/ERK MAPK pathway in PE-induced cardiomyocyte hypertrophy
To further identify the upstream regulatory pathway of PE-induced PARP16 upregulation, we examined mitogen-activated protein kinase (MAPK) signaling pathway, a classical pathway involved in cardiac hypertrophy [20]. Our results showed that the phosphorylation of MAPK pathway members, including JNK, ERK1/2, except p38 MAPK were increased upon PE challenge in vitro (Fig. S6a). Then the specific inhibitors of JNK, ERK1/2 and p38 MAPK, namely SP600125, PD98059 and SB203580 were used and found the expression of PARP16 was inhibited by JNK and ERK1/2 inhibitors but not p38 MAPK inhibitors (Fig. S6b), suggesting that PE-induced PARP16 upregulation maybe mediated by JNK/ERK MAPK pathway.
Discussion
Pathological cardiac hypertrophy is regarded as an independent risk factor of HF, whose current main therapeutic interventions are still a bottleneck problem [1]. Thus, a deeper understanding of the mechanisms involved in pathological cardiac hypertrophy is necessary for exploring the potential drug targets. In the present study, we determined that PARP16 was a key driver of pathological cardiac hypertrophy. PARP16 deficiency rescued cardiac dysfunction and ameliorated TAC-induced cardiac hypertrophy and fibrosis in mice, as well as PE-induced cardiomyocyte hypertrophic responses. Whereas overexpression of PARP16 exacerbated the hypertrophic responses. Mechanistically, PARP16 interacted with IRE1α and ADP-ribosylated IRE1α and mediated the hypertrophic responses through activating the IRE1α–sXBP1–GATA4 pathway. Collectively, our results implicated that PARP16 may be a contributor of pathological cardiac hypertrophy at least partly via activating the IRE1α–sXBP1–GATA4 pathway, and may be regarded as a new potential target for exploring effective therapeutic interventions of pathological cardiac hypertrophy and HF.
The poly (ADP-ribose) polymerases (PARPs) family members such as PARP-1 and PARP-2 has been revealed to participate in the progression of cardiac hypertrophy due to different cellular localization and regulating mechanisms. PARP-1 contributes to caspase-independent myocyte cell death during heart failure [21] while knockdown of PARP-2 protects against cardiac hypertrophy via SIRT1 activation [22]. However, PARP16 is the only PARP family member to be located on the endoplasmic reticulum and its role in cardiovascular diseases remains to be clarified. Recently, emerging evidence has demonstrated that PARP16 is identified as a novel target for vascular diseases, such as vascular aging and neointimal hyperplasia related diseases [11, 12]. Consistent with this, we were curious about the effect of PARP16 on pathological cardiac hypertrophy and observed PARP16 was at a higher level in response to pressure overload-induced pathological cardiac hypertrophy. PARP16 deficiency rescued cardiac dysfunction and ameliorated TAC-induced cardiac hypertrophy and fibrosis as well as PE-induced cardiomyocyte hypertrophic responses. Whereas overexpression of PARP16 exacerbated the hypertrophic responses, implicating that PARP16 may be a contributor of pathological cardiac hypertrophy.
It is also well documented that GATA transcription factors have important roles to promote ER integrity and Gata4 and Gata6 knockout mice could lead to several ER stress signals activation, such as CHOP [23]. However, the relationship between ER stress and GATA4 hasn't been explained well in pathological cardiac hypertrophy. In our study, we provided evidence that PARP16 could regulate the transcription expression of GATA4 to some extent depending on the IRE1α–sXBP1 pathway by using two molecular inhibitors (TUDCA, an ER stress inhibitor and STF-083010, a specific IRE1α inhibitor) as well as IRE1α siRNA. Indeed, treatment with either TUDCA or STF-083010 could blunt the expression of GATA4 in response to PE or PARP16 OE treatment. Furthermore, knockdown of IRE1α with siRNA significantly decreased the protein expression of GATA4 and BNP in PE-induced NRCMs, suggesting that PARP16 mediated expression of GATA4 at least in part via the IRE1α–sXBP1 pathway. Moreover, GATA4 acts as a key zinc finger-containing transcription factor of numerous cardiac-specific genes including Nppa and Nppb [16, 24] and ablation of GATA4 by siRNAs decreased ANP expression [25], therefore PARP16 contribute to the upregulation of ANP and BNP at least partly via activating the IRE1α–sXBP1–GATA4 pathway, further promote the progress of cardiac hypertrophy.
Due to the limited clinic resources, further verifications were not carried out in human samples, which is the deficiency of this study. Another limitation of this study are that we did not construct cardiac-specific knockout mice or cardiac-specific targeting adenoviruses, which should be explored in future studies.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- AAV:
-
Adeno-associated virus
- AAV9-shPARP16:
-
Serotype 9 AAV vectors encoding mouse PARP16 shRNA
- ACEIs:
-
Angiotensin converting enzyme inhibitors
- Ang II:
-
Angiotensin II
- ATF6:
-
Activating transcription factor 6
- BrdU:
-
Bromodeoxyuridine
- Co-IP:
-
Co-immunoprecipitation
- DAPI:
-
4, 6-Diamidino-2-phenylindole
- EF:
-
Ejection fraction
- EGCG:
-
Epigallocatechin-3-gallate
- FBS:
-
Fetal bovine serum
- FS:
-
Fractional shortening
- H&E:
-
Hematoxylin and eosin
- H3K4:
-
Histone H3 lysine 4
- HF:
-
Heart failure
- HW/TL:
-
Heart weight/tibia length
- IHC:
-
Immunohistochemistry
- IRE1α:
-
Inositol requiring enzyme 1α
- IVSd:
-
The interventricular septal thickness at diastole
- IVSs:
-
The interventricular septal thickness at systole
- LV:
-
Left ventricular
- LVESV:
-
LV end-systolic volume
- LVID; s:
-
LV internal dimension at systole
- MAPK:
-
Mitogen-activated protein kinase
- NC:
-
Nitrocellulose
- NRCMs:
-
Neonatal rat cardiomyocytes
- PARP:
-
Poly (ADP-ribose) polymerase
- PARP16 OE:
-
PARP16 overexpression lentivirus
- PE:
-
Phenylephrine
- PERK:
-
Protein kinase R-like ER kinase
- RT:
-
Room temperature
- RT-qPCR:
-
Real-time and quantitative PCR
- SD:
-
Sprague–Dawley
- shCTL:
-
Lentivirus scramble shRNA
- shPARP16:
-
Lentivirus PARP16 shRNA
- siIRE1α:
-
IRE1α-specific short interfering RNA
- siPARP16:
-
PARP16-specific short interfering RNA
- sXBP1:
-
Spliced XBP1
- TAC:
-
Transverse aortic construction
- TUDCA:
-
Tauroursodeoxycholic acid
- UPR:
-
Unfolded protein response
- uXBP1:
-
Un-spliced XBP1
- WGA:
-
Wheat germ agglutinin
References
Ren Z, Yu P, Li D, Li Z, Liao Y, Wang Y et al (2020) Single-cell reconstruction of progression trajectory reveals intervention principles in pathological cardiac hypertrophy. Circulation 141(21):1704–1719. https://doi.org/10.1161/CIRCULATIONAHA.119.043053
Frey N, Olson EN (2003) Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 65:45–79. https://doi.org/10.1146/annurev.physiol.65.092101.142243
Wang ZV, Hill JA (2015) Protein quality control and metabolism: bidirectional control in the heart. Cell Metab 21(2):215–226. https://doi.org/10.1016/j.cmet.2015.01.016
Blackwood EA, Hofmann C, Santo Domingo M, Bilal AS, Sarakki A, Stauffer W et al (2019) ATF6 regulates cardiac hypertrophy by transcriptional induction of the mTORC1 activator, Rheb. Circ Res 124(1):79–93. https://doi.org/10.1161/CIRCRESAHA.118.313854
Yang L, Dai R, Wu H, Cai Z, **e N, Zhang X et al (2022) Unspliced XBP1 counteracts beta-catenin to inhibit vascular calcification. Circ Res 130(2):213–229. https://doi.org/10.1161/CIRCRESAHA.121.319745
Zhang Y, Chen W, Wang Y (2020) STING is an essential regulator of heart inflammation and fibrosis in mice with pathological cardiac hypertrophy via endoplasmic reticulum (ER) stress. Biomed Pharmacother 125:110022. https://doi.org/10.1016/j.biopha.2020.110022
Duan Q, Chen C, Yang L, Li N, Gong W, Li S et al (2015) MicroRNA regulation of unfolded protein response transcription factor XBP1 in the progression of cardiac hypertrophy and heart failure in vivo. J Transl Med 13:363. https://doi.org/10.1186/s12967-015-0725-4
Jwa M, Chang P (2012) PARP16 is a tail-anchored endoplasmic reticulum protein required for the PERK- and IRE1alpha-mediated unfolded protein response. Nat Cell Biol 14(11):1223–1230. https://doi.org/10.1038/ncb2593
Palve V, Knezevic CE, Bejan DS, Luo Y, Li X, Novakova S et al (2022) The non-canonical target PARP16 contributes to polypharmacology of the PARP inhibitor talazoparib and its synergy with WEE1 inhibitors. Cell Chem Biol 29(2):202-214.e7. https://doi.org/10.1016/j.chembiol.2021.07.008
Wang J, Zhu C, Song D, **a R, Yu W, Dang Y et al (2017) Epigallocatechin-3-gallate enhances ER stress-induced cancer cell apoptosis by directly targeting PARP16 activity. Cell Death Discov 3:17034. https://doi.org/10.1038/cddiscovery.2017.34
Long F, Yang D, Wang J, Wang Q, Ni T, Wei G et al (2021) SMYD3-PARP16 axis accelerates unfolded protein response and mediates neointima formation. Acta Pharm Sin B 11(5):1261–1273. https://doi.org/10.1016/j.apsb.2020.12.010
Yang D, Wang Q, Wei G, Wu J, Zhu YC, Zhu Q et al (2020) Smyd3-PARP16 axis accelerates unfolded protein response and vascular aging. Aging 12(21):21423–21445. https://doi.org/10.18632/aging.103895
Yang D, Wei G, Long F, Nie H, Tian X, Qu L et al (2020) Histone methyltransferase Smyd3 is a new regulator for vascular senescence. Aging Cell 19(9):e13212. https://doi.org/10.1111/acel.13212
Lunde IG, Kvaloy H, Austbo B, Christensen G, Carlson CR (2011) Angiotensin II and norepinephrine activate specific calcineurin-dependent NFAT transcription factor isoforms in cardiomyocytes. J Appl Physiol 111(5):1278–1289. https://doi.org/10.1152/japplphysiol.01383.2010
Yao Y, Lu Q, Hu Z, Yu Y, Chen Q, Wang QK (2017) A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat Commun 8(1):133. https://doi.org/10.1038/s41467-017-00171-w
Akazawa H, Komuro I (2003) Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res 92(10):1079–1088. https://doi.org/10.1161/01.RES.0000072977.86706.23
Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA et al (2006) Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res 98(6):837–845. https://doi.org/10.1161/01.RES.0000215985.18538.c4
Bis** E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR et al (2006) Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci USA 103(39):14471–14476. https://doi.org/10.1073/pnas.0602543103
Molkentin JD (2000) The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem 275(50):38949–38952. https://doi.org/10.1074/jbc.R000029200
Shen Y, Zhang X, Li C, Wang X, Ye Y, Yuan J et al (2020) Pressure overload promotes cystatin C secretion of cardiomyocytes to regulate the MAPK signaling pathway and mediate cardiac hypertrophy. Ann Transl Med 8(22):1514. https://doi.org/10.21037/atm-20-7041
Pillai JB, Russell HM, Raman J, Jeevanandam V, Gupta MP (2005) Increased expression of poly(ADP-ribose) polymerase-1 contributes to caspase-independent myocyte cell death during heart failure. Am J Physiol Heart Circ Physiol 288(2):H486–H496. https://doi.org/10.1152/ajpheart.00437.2004
Biao Geng YC, Gao S, Lu J, Zhang L, Zou J, Liu M, Yu S, Ye J, Liu P (2013) PARP-2 knockdown protects cardiomyocytes from hypertrophy via activation of SIRT1. Biochem Biophys Res Commun 430(3):944–950. https://doi.org/10.1016/j.bbrc.2012.11.132
Sartori DJ, Wilbur CJ, Long SY, Rankin MM, Li C, Bradfield JP et al (2014) GATA factors promote ER integrity and beta-cell survival and contribute to type 1 diabetes risk. Mol Endocrinol 28(1):28–39. https://doi.org/10.1210/me.2013-1265
Zhang A, David JJ, Subramanian SV, Liu X, Fuerst MD, Zhao X et al (2008) Serum response factor neutralizes Pur alpha- and Pur beta-mediated repression of the fetal vascular smooth muscle alpha-actin gene in stressed adult cardiomyocytes. Am J Physiol Cell Physiol 294(3):C702–C714. https://doi.org/10.1152/ajpcell.00173.2007
Sun S, Li T, ** L, Piao ZH, Liu B, Ryu Y et al (2018) Dendropanax morbifera prevents cardiomyocyte hypertrophy by inhibiting the Sp1/GATA4 pathway. Am J Chin Med 46(5):1021–1044. https://doi.org/10.1142/S0192415X18500532
Acknowledgements
We are grateful to prof. **nhua Liu for expertise, assistance and productive critiques.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 81903602), Shanghai Municipal Science and Technology Major Project (Grant No. 2017SHZDZX01), innovative research team of high-level local universities in Shanghai and a key laboratory program of the Education Commission of Shanghai Municipality (ZDSYS14005) and Macau Science and Technology Development fund (0007/2019/AKP, 0144/2022/A3).
Author information
Authors and Affiliations
Contributions
Study designment, SHB and YD; experiments performance, SHB, XJ, SZH and XCX; data interpretation and analysis, YD, SHB, XCX, WJH and ZW; manuscript composition, YD and SHB; manuscript revision, SHB, XJ, WJH and MC; making suggestions for revision, ZYZ and YD. All authors have read the content of this manuscript and agreed to publish it.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare that they have no confilct of interest.
Ethics statement of animal studies
All the experimental procedures involving animals were conformed to the Animal Welfare Act Guide for Use and Care of Laboratory Animals, and were approved by Institutional Animal Care and Use Committee (IACUC), Fudan University, China. All animals were housed under specific pathogen-free (SPF) conditions at a stable temperature of 24 °C and fed with chow diet and water at libitum. The male mice were chosen because of the variables (uncertain hormone changes et al.) in female mice. The mice were euthanized by intraperitoneal (i.p.) administration of pentobarbital sodium (50 mg/kg body weight) then the hearts were harvested for further examination.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Su, H., Xu, J., Su, Z. et al. Poly (ADP-ribose) polymerases 16 triggers pathological cardiac hypertrophy via activating IRE1α–sXBP1–GATA4 pathway. Cell. Mol. Life Sci. 80, 161 (2023). https://doi.org/10.1007/s00018-023-04805-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04805-9