Abstract
Objective
Ferroptosis is a reactive oxygen species (ROS)- and iron-dependent form of non-apoptotic cell death process. Previous studies have demonstrated that ferroptosis participates in the development of inflammatory arthritis. However, the role of ferroptosis in rheumatoid arthritis (RA) inflammatory hypoxic joints remains unclear. This study sought to explore the underlying mechanism of ferroptosis on lipopolysaccharide (LPS)-induced RA fibroblast-like synoviocytes (FLSs).
Methods
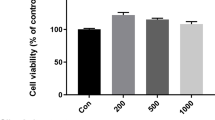
FLSs, isolated from patients with RA, were treated with LPS and ferroptosis inducer (erastin and RSL-3), and ferroptosis inhibitor (Fer-1 and DFO), respectively. The cell viability was measured by CCK-8. The cell death was detected by flow cytometer. The proteins level were tested by Western blot. The cytosolic ROS and lipid peroxidation were determined using DCFH-DA and C11-BODIPY581/591 fluorescence probes, respectively. The small interfering RNA (siRNA) was used to knock down related proteins. The levels of malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), iron, inflammatory cytokines (IL6 and IL8), and LDH were analyzed by commercial kits.
Results
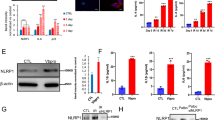
Ferroptosis was activated by LPS in RA FLS with increased cellular damage, ROS and lipid peroxidation, intracellular Fe and IL8, which can be further amplified by ferroptosis inducer (erastin and RSL-3) and inhibited by ferroptosis inhibitor (Fer-1 and DFO). Mechanistically, LPS triggered ferroptosis via NCOA4-mediated ferritinophagy in RA FLSs, and knockdown of NCOA4 strikingly prevent the process of ferroptosis. Intriguingly, LPS-induced RA FLSs became insensitive to ferroptosis and NCOA4-mediated ferritinophagy under hypoxia compared with normoxia. Knockdown of HIF-1α reverted ferroptosis and ferritinophagy evoking by LPS-induced RA FLSs inflammation under hypoxia. In addition, low dose of auranofin (AUR) induced re-sensitization of ferroptosis and ferritinophagy through inhibiting the expression of HIF-1α under hypoxia.
Conclusions
NCOA4-mediated ferritinophagy was a key driver of ferroptosis in inflammatory RA FLSs. The suppression of NCOA4-mediated ferritinophagy protected RA FLSs from ferroptosis in LPS-induced inflammation under hypoxia. Targeting HIF-1α/NCOA4 and ferroptosis could be an effective and valuable therapeutic strategy for synovium hyperplasia in the patients with RA.







Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- 4-HNE:
-
4-Hydroxynonenal
- AUR:
-
Auranofin
- CA9:
-
Carbonic anhydrase 9
- DFO:
-
Deferoxamine mesylate
- Fer-1:
-
Ferrostatin-1
- FLSs:
-
Fibroblast-like synoviocytes
- FPN:
-
Ferroportin
- FTH:
-
Ferritin heavy chain
- FTL:
-
Ferritin light chain
- HIF-1:
-
Hypoxia-inducible factor-1
- LPS:
-
Lipopolysaccharide
- MDA:
-
Malondialdehyde
- NCOA4:
-
Nuclear receptor coactivator 4
- PDAC:
-
Pancreatic ductal adenocarcinomas
- RA:
-
Rheumatoid arthritis
- ROS:
-
Reactive oxygen species
- siRNA:
-
Small interfering RNA
- SLE:
-
Systemic lupus erythematosus
- TfR:
-
Transferrin receptor
References
Peters CL, Morris CJ, Mapp PI, Blake DR, Lewis CE, Winrow VR. The transcription factors hypoxia-inducible factor 1alpha and Ets-1 colocalize in the hypoxic synovium of inflamed joints in adjuvant-induced arthritis. Arthritis Rheum. 2004;50:291–6.
Konisti S, Kiriakidis S, Paleolog EM. Hypoxia–a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8(3):153–62.
Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–96.
Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11(7):3052–9.
Wang Y, Quan F, Cao Q, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2020;28:231–43.
Xu S, He Y, Lin L, Chen P, Chen M, Zhang S. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021;12(4):289.
Ou M, Jiang Y, Ji Y, et al. Role and mechanism of ferroptosis in neurological diseases. Mol Metab. 2022;61: 101502.
Li P, Jiang M, Li K, et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat Immunol. 2021;22(9):1107–17.
Luoqian J, Yang W, Ding X, et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell Mol Immunol. 2022;19(8):913–24.
Zhou R, Chen Y, Li S, et al. TRPM7 channel inhibition attenuates rheumatoid arthritis articular chondrocyte ferroptosis by suppression of the PKCα-NOX4 axis. Redox Biol. 2022;55: 102411.
McGettrick AF, O’Neill LAJ. The role of HIF in immunity and inflammation. Cell Metab. 2020;32(4):524–36.
Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17.
Cen Y, Wang P, Gao F, et al. Tetramethylpyrazine nitrone activates hypoxia-inducible factor and regulates iron homeostasis to improve renal anemia. Front Pharmacol. 2022;13:964234.
Ni S, Yuan Y, Qian Z, et al. Hypoxia inhibits RANKL-induced ferritinophagy and protects osteoclasts from ferroptosis. Free Radic Biol Med. 2021;169:271–82.
Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–9.
Ryu MS, Duck KA, Philpott CC. Ferritin iron regulators, PCBP1 and NCOA4, respond to cellular iron status in develo** red cells. Blood Cells Mol Dis. 2018;69:75–81.
Dowdle WE, Nyfeler B, Nagel J, et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16(11):1069–79.
Dong W, Tan Y, Qin Q, et al. Polybrominated diphenyl ethers quinone induces NCOA4-mediated ferritinophagy through selectively autophagic degradation of ferritin. Chem Res Toxicol. 2019;32(12):2509–16.
Li Q, Chen H, Huang X, Costa M. Effects of 12 metal ions on iron regulatory protein 1 (IRP-1) and hypoxia-inducible factor-1 alpha (HIF-1alpha) and HIF-regulated genes. Toxicol Appl Pharmacol. 2006;213(3):245–55.
Suarez-Almazor ME, Spooner CH, Belseck E. Shea B (2000) Auranofin versus placebo in rheumatoid arthritis. Cochrane Database Syst Rev. 2000;2:CD002048.
Freire Boullosa L, Van Loenhout J, Flieswasser T, et al. Auranofin reveals therapeutic anticancer potential by triggering distinct molecular cell death mechanisms and innate immunity in mutant p53 non-small cell lung cancer. Redox Biol. 2021;42:101949.
Van Loenhout J, Freire Boullosa L, Quatannens D, et al. Auranofin and cold atmospheric plasma synergize to trigger distinct cell death mechanisms and immunogenic responses in glioblastoma. Cells. 2021;10(11):2936.
Kato I, Kasukabe T, Kumakura S. Menin-MLL inhibitors induce ferroptosis and enhance the anti-proliferative activity of auranofin in several types of cancer cells. Int J Oncol. 2020;57(4):1057–71.
You S, Koh JH, Leng L, Kim WU, Bucala R. The tumor-like phenotype of rheumatoid synovium: molecular profiling and prospects for precision medicine. Arthritis Rheumatol. 2018;70(5):637–52.
Liu Y, Wei W, Wang Y, et al. TNF-α/calreticulin dual signaling induced NLRP3 inflammasome activation associated with HuR nucleocytoplasmic shuttling in rheumatoid arthritis. Inflamm Res. 2019;68(7):597–611.
Luo H, Zhang R. Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis. Exp Ther Med. 2021;21(1):72.
Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72.
Van der Paal J, Neyts EC, Verlackt CCW, Bogaerts A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem Sci. 2016;7(1):489–98.
Gryzik M, Srivastava A, Longhi G, et al. Expression and characterization of the ferritin binding domain of nuclear receptor coactivator-4 (NCOA4. Biochim Biophys Acta Gen Subj. 2017;1861((11 Pt A)):2710–6.
Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36:101670.
Lin Z, Song J, Gao Y, et al. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022;52:102312.
Mao H, Zhao Y, Li H, Lei L. Ferroptosis as an emerging target in inflammatory diseases. Prog Biophys Mol Biol. 2020;155:20–8.
Wu J, Feng Z, Chen L, et al. TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat Commun. 2022;13(1):676.
Ling H, Li M, Yang C, et al. Glycine increased ferroptosis via SAM-mediated GPX4 promoter methylation in rheumatoid arthritis. Rheumatology (Oxford). 2022;61(11):4521–34.
Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15(8):500–10.
Tefanova KI, Delcheva GT, Maneva AI, et al. Pathobiochemical mechanisms relating iron homeostasis to parameters of inflammatory activity and autoimmune disorders in rheumatoid arthritis. Folia Med (Plovdiv). 2016;58(4):257–63.
**e Z, Hou H, Luo D, An R, Zhao Y, Qiu C. ROS-dependent lipid peroxidation and reliant antioxidant ferroptosis-suppressor-protein 1 in rheumatoid arthritis: a covert clue for potential therapy. Inflammation. 2021;44(1):35–47.
Mancias JD, Pontano Vaites L, Nissim S, et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife. 2015;4:e10308.
Santana-Codina N, Del Rey MQ, Kapner KS, et al. NCOA4-mediated ferritinophagy is a pancreatic cancer dependency via maintenance of iron bioavailability for iron-sulfur cluster proteins. Cancer Discov. 2022;12(9):2180–97.
Li N, Wang W, Zhou H, et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med. 2020;160:303–18.
Feng X, Wang S, Sun Z, et al. Ferroptosis enhanced diabetic renal tubular Injury via HIF-1α/HO-1 pathway in db/db Mice. Front Endocrinol (Lausanne). 2021;12:626390.
Yuan S, Wei C, Liu G, et al. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell Prolif. 2022;55(1):e13158.
Singhal R, Mitta SR, Das NK, et al. HIF-2α activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J Clin Invest. 2021;131(12):e143691.
Wang Y, Zhang L, Zhou X. Activation of Nrf2 signaling protects hypoxia-induced HTR-8/SVneo cells against ferroptosis. J Obstet Gynaecol Res. 2021;47(11):3797–806.
Liu XJ, Lv YF, Cui WZ, et al. Icariin inhibits hypoxia/reoxygenation-induced ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1 signaling pathway. FEBS Open Bio. 2021;11(11):2966–76.
Li Z, Jiang L, Chew SH, Hirayama T, Sekido Y, Toyokuni S. Carbonic anhydrase 9 confers resistance to ferroptosis/apoptosis in malignant mesothelioma under hypoxia. Redox Biol. 2019;26:101297.
Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154(2):204–19.
Yumnamcha T, Devi TS, Singh LP. Auranofin mediates mitochondrial dysregulation and inflammatory cell death in human retinal pigment epithelial cells: implications of retinal neurodegenerative diseases. Front Neurosci. 2019;13:1065.
Yang L, Wang H, Yang X, et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct Target Ther. 2020;5(1):138.
Gnanapradeepan K, Basu S, Barnoud T, Budina-Kolomets A, Kung CP, Murphy ME. The p53 tumor suppressor in the control of metabolism and ferroptosis. Front Endocrinol (Lausanne). 2018;9:124.
Hassannia B, Vandenabeele P, Vanden BT. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830–49.
Liu J, Zhang C, Wang J, Hu W, Feng Z. The regulation of ferroptosis by tumor suppressor p53 and its pathway. Int J Mol Sci. 2020;21(21):8387.
Rios Perez MV, Roife D, Dai B, et al. Antineoplastic effects of auranofin in human pancreatic adenocarcinoma preclinical models. Surg Open Sci. 2019;1(2):56–63.
Acknowledgements
This work was supported by Special Fund for Discipline Construction of Tian** Medical University during the 13th Five-year Plan Period (2017XK030605; to F.Z), Natural Science Foundation of Tian** (20JCYBJC00700; to F.Z), and Integrative Medicine Foundation of Tian** Administration of Traditional Chinese Medicine (2021030; to H.D).
Funding
Integrative Medicine Foundation of Tian** Administration of Traditional Chinese Medicine, 2021030, Natural Science Foundation of Tian** Municipality, 20JCYBJC00700.
Author information
Authors and Affiliations
Contributions
Y W and H M D contributed to conceptualization, methodology, investigation, and writing––review and editing. Y Q Z was responsible for investigation, methodology, and software. X Y W and X T Y were involved in conceptualization, methodology, and writing––review and editing. H W performed data curation and writing––review and editing. Y S T was responsible for data curation, methodology, and writing––review and editing. W W was involved in methodology and supervision. J M performed supervision and project administration. F Z and D R T performed funding acquisition, supervision, and project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Ding, H., Zheng, Y. et al. Alleviated NCOA4-mediated ferritinophagy protected RA FLSs from ferroptosis in lipopolysaccharide-induced inflammation under hypoxia. Inflamm. Res. 73, 363–379 (2024). https://doi.org/10.1007/s00011-023-01842-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01842-9