Abstract
Objectives
Accurate and timely diagnosis and prognosis of sepsis remain challenging. A combination of markers, as opposed to single ones, may improve the prognosis, and therefore survival. This study compared the effectiveness of routinely used biomarkers of sepsis alone and in combination for the prediction of outcome in rats with abdominal sepsis.
Methods
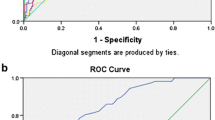
Rats were subjected to sepsis induced by cecal ligation and puncture (CLP). Seventeen biomarkers were detected 12 h after the CLP. Correlation between the biomarkers and outcome of rats was analyzed; the correlated biomarkers were analyzed by logistic regression analysis and the area under the receiver operating characteristic (ROC) curve was computed to compare their performance in the prognosis of sepsis.
Results
A total of 49 rats were eligible for analysis. Body temperature (T), blood urea nitrogen (BUN), creatinine (Cr), alanine aminotransferase (ALT), creatine kinase (CK), interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) levels after the CLP were negatively correlated with the survival outcome, while platelet count (PLT), high-mobility group box protein 1 (HMGB1) and granulocyte–macrophage colony stimulating factor (GM-CCF) were positively correlated with the survival outcome (P < 0.05). Levels of BUN, Cr, IL-6, and GM-CSF after the CLP were independent predictors of outcome according to conditional logistic regression. The sensitivity and specificity of the four selected biomarkers in combination for predicting sepsis outcome were better than single ones (P < 0.05).
Conclusion
A combination of different biomarkers improves the diagnostic accuracy and is more effective in the prognosis of sepsis in rats. Use of BUN, Cr, IL-6, GM-CSF in combination to predict the severity and outcome in rats with abdominal sepsis exhibited acceptable diagnostic characteristics.




Similar content being viewed by others
References
Alberta C, Brun-Buisson C, Goodman SV, Guidici D, Granton J, Moreno R, et al. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med. 2003;168:77–84.
Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335:879–83.
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Meisner M. Biomarkers of sepsis: clinically useful? Curr Opin Crit Care. 2005;11:473–80.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6.
Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15.
Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–10.
Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538–46.
Sakr Y, Vincent JL, Schuerholz T, Filipescu D, Romain A, Hjelmqvist H, et al. Early-versus late-onset shock in European intensive care units. Shock. 2007;28:636–43.
Lin SM, Frevert CW, Kajikawa O, Wurfel MM, Ballman K, Mongovin S, et al. Differential regulation of membrane CD14 expression and endotoxin-tolerance in alveolar macrophages. Am J Respir Cell Mol Biol. 2004;31:162–70.
Holly MK, Dear JW, Hu X, Schechter AN, Gladwin MT, Hewitt SM, et al. Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int. 2006;70:496–506.
Heresi GA. Acute renal failure and sepsis. N Engl J Med 2004; 351:2347-9; author reply 2347-9.
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50.
Patel RT, Deen KI, Youngs D, Warwick J, Keighley MR. Interleukin 6 is a prognostic indicator of outcome in severe intra-abdominal sepsis. Br J Surg. 1994;81:1306–8.
Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab,)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173–82.
Gogos CA, Skoutelis A, Lekkou A, Drosou E, Starakis I, Marangos MN, et al. Comparative effects of ciprofloxacin and ceftazidime on cytokine production in patients with severe sepsis caused by gram-negative bacteria. Antimicrob Agents Chemother. 2004;48:2793–8.
Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamua M. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine. 2005;29:169–75.
Yoon DY, Chu J, Chandler C, Hiyama S, Thompson JE, Hines OJ. Human cytokine levels in nonperforated versus perforated appendicitis: molecular serum markers for extent of disease? Am Surg. 2002;68:1033–7.
Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73:2751–7.
Hamilton JA, Anderson GP. GM-CSF Biology. Growth Factors. 2004;22:225–31.
Presneill JJ, Waring PM, Layton JE, Maher DW, Cebon J, Harley NS, et al. Plasma granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor levels in critical illness including sepsis and septic shock: relation to disease severity, multiple organ dysfunction, and mortality. Crit Care Med. 2000;28:2344–54.
Perry SE, Mostafa SM, Wenstone R, McLaughlin PJ. Low plasma granulocyte-macrophage colony stimulating factor is an indicator of poor prognosis in sepsis. Intensive Care Med. 2002;28:981–4.
Nierhaus A, Montag B, Timmler N, Frings DP, Gutensohn K, Jung R, et al. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med. 2003;29:646–51.
Kylanpaa ML, Mentula P, Kemppainen E, Puolakkainen P, Aittomaki S, Silvennoinen O, et al. Monocyte anergy is present in patients with severe acute pancreatitis and is significantly alleviated by granulocyte-macrophage colony-stimulating factor and interferon-gamma in vitro. Pancreas. 2005;31:23–7.
Acknowledgments
The authors would like to thank Weixin Hu for his kind assistance in statistical analysis. This research was supported by National Natural Science Foundation of China (81071594).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Gao, M., Zhang, L., Liu, Y. et al. Use of blood urea nitrogen, creatinine, interleukin-6, granulocyte–macrophage colony stimulating factor in combination to predict the severity and outcome of abdominal sepsis in rats. Inflamm. Res. 61, 889–897 (2012). https://doi.org/10.1007/s00011-012-0481-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-012-0481-3