Abstract
Objective
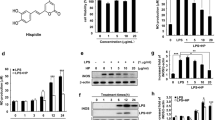
We have previously synthesized a novel piperidine compound, 3-[(dodecylthiocarbonyl)methyl]glutarimide (DTCM-glutarimide), that inhibits LPS-induced NO production, and in the present research we studied further the anti-inflammatory activity of DTCM-glutarimide in a macrophage cell line and in mice bearing transplanted hearts.
Materials and methods
Mouse macrophage-like RAW264.7 cells were employed for the evaluation of cellular inflammatory activity. DTCM-glutarimide was synthesized in our laboratory. The AP-1 activity was measured by nuclear translocation and phosphorylation. For the heart transplantation experiment, male C57BL/6 (H-2b) and BALB/c (H-2d) mice were used as donor and recipient, respectively. DTCM-glutarimide was administered intraperitoneally.
Results
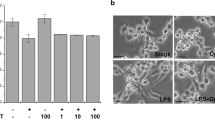
DTCM-glutarimide inhibited the LPS-induced expression of iNOS and COX-2 in macrophages; but, unexpectedly, it did not inhibit LPS-induced NF-κB activation. Instead, it inhibited the nuclear translocation of both c-Jun and c-Fos. It also inhibited LPS-induced c-Jun phosphorylation. Moreover, it inhibited the mixed lymphocyte reaction in primary cultures of mouse spleen cells; and furthermore, in mice it prolonged the graft survival in heart transplantation experiments.
Conclusion
The novel piperidine compound, DTCM-glutarimide, was found to be a new inhibitor of macrophage activation, inhibiting AP-1 activity. It also inhibited graft rejection in mice, and thus may be a candidate for an anti-inflammatory agent.





Similar content being viewed by others
References
Matsumoto N, Ariga A, To-e S, Nakamura H, Agata N, Hirano S, et al. Synthesis of NF-kappaB activation inhibitors derived from epoxyquino- micin C. Bioorg Med Chem Lett. 2000;10:865–9.
Ariga A, Namekawa J, Matsumoto N, Inoue J, Umezawa K. Inhibition of tumor necrosis f actor-alpha-induced nuclear translocation and activation of NF-kappa B by dehydroxy-methylepoxyquinomicin. J Biol Chem. 2002;277:24625–30.
Yamamoto M, Horie R, Takeiri M, Kozawa I, Umezawa K. Inactivation of NF-kappaB components by covalent binding of (−)-dehydroxymethylepoxyquinomicin to specific cysteine residues. J Med Chem. 2008;51:5780–8.
Watanabe M, Ohsugi T, Shoda M, Ishida T, Aizawa S, Maruyama-Nagai M, Utsunomiya A, Koga S, Yamada Y, Kamihira S, Okayama A, Kikuchi H, Uozumi K, Yamaguchi K, Higashihara M, Umezawa K, Watanabe T, Horie R. Dual targeting of transformed and untransformed HTLV-1-infected T-cells by DHMEQ, a potent and selective inhibitor of NF-κB, as a strategy for chemoprevention and therapy of adult T cell leukemia. Blood. 2005;106:2462–71.
Umezawa K. Inhibition of tumor growth by NF-κB inhibitors. Cancer Sci. 2006;97:990–5.
Hamasaka A, Yoshioka N, Abe R, Kishino S, Umezawa K, Ozaki M, Todo S, Shimizu H. Topical application of DHMEQ improves allergic inflammation via NF-κB inhibition. J Allergy Clin Immunol. 2010;126:400–3.
Ueki S, Yamashita K, Aoyagi T, Haga S, Suzuki T, Itoh T, et al. Control of allograft rejection by applying a novel nuclear factor-kappaB inhibitor, dehydroxymethylepoxy-quinomicin. Transplantation. 2006;82:1720–7.
Umezawa K. Screening of bioactive metabolites that suppress inflammation. Tanpakushitsu Kakusan Koso. 2007;52:1685–9.
Ishikawa Y, Tachibana M, Matsui C, Obata R, Umezawa K, Nishiyama S. Synthesis and biological evaluation on novel analogs of 9-methylstreptimidone, an inhibitor of NF-kappaB. Bioorg Med Chem Lett. 2009;19:1726–8.
Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–89.
Corry RJ, Winn HJ, Russel PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–50.
Nomura M, Yamashita K, Murakami M, Takehara M, Echizenya H, Sunahara M, et al. Induction of donor-specific tolerance by adenovirus-mediated CD40Ig gene therapy in rat liver transplantation. Transplantation. 2002;73(9):1403–10.
Jang JH, Surh YJ. AP-1 mediates beta-amyloid-induced iNOS expression in PC12 cells via the ERK2 and p38 MAPK signaling pathways. Biochem Biophys Res Commun. 2005;331:1421–8.
Yea SS, Jeong HS, Choi CY, Park KR, Oh S, Shin JG, et al. Inhibitory effect of anethole on T-lymphocyte proliferation and interleukin-2 production through down-regulation of the NF-AT and AP-1. Toxicol In Vitro. 2006;20:1098–105.
Thornton TM, Zullo AJ, Williams KL, Taparowsky EJ. Direct manipulation of activator protein-1 controls thymocyte proliferation in vitro. Eur J Immunol. 2006;36:160–9.
Park PH, Kim HS, ** XY, ** F, Hur J, Ko G, et al. KB-34, a newly synthesized chalcone derivative, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via heme oxygenase-1 induction and blockade of activator protein-1. Eur J Pharmacol. 2009;606:215–24.
Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007;81:1602–14.
Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–60.
Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–40.
Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120.
Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73.
Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–4.
Yao J, Mackman N, Edgington TS, Fan ST. Inhibitory effect of 1,8-cineol (eucalyptol) on Egr-1 expression in lipopolysaccharide-stimulated THP-1 cells. J Biol Chem. 1997;272:17795–801.
Nakabeppu Y, Ryder K, Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988;5:907–15.
Ryseck RP, Kovary K, Bravo R. Integrity of FOS B leucine zipper is essential for its interaction with JUN proteins. Oncogene. 1990;5:1091–3.
Cohen DR, Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988;8:2063–9.
Nishina H, Sato H, Suzuki T, Sato M, Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci USA. 1990;87:3619–23.
Chiu R, Boyle WJ, Meek J, Smeal T, Hunter T, Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988;54:541–52.
Macgregor PF, Abate C, Curran T. Direct cloning of leucine zipper proteins: Jun binds cooperatively to the CRE with CRE-BP1. Oncogene. 1990;5:451–8.
Hsu JC, Laz T, Mohn KL, Taub R. Identification of LRF-1, a leucine-zipper protein that is rapidly and highly induced in regenerating liver. Proc Natl Acad Sci USA. 1991;88:3511–5.
Dorsey MJ, Tae HJ, Sollenberger KG, Mascarenhas NT, Johansen LM, Taparowsky EJ. B-ATF: a novel human bZIP protein that associates with members of the AP-1 transcription factor family. Oncogene. 1995;11:2255–65.
Dérijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37.
Aikawa Y, Morimoto K, Yamamoto T, Chaki H, Hashiramoto A, Narita H, Hirono S, Shiozawa S. Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1. Nat Biotechnol. 2008;26:817–23.
Acknowledgments
This work was financially supported in part by grants from the program Grants-in-Aid for Scientific Research on Priority Areas of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT). It was also supported by a High-Tech Research Center Project for Private Universities: matching fund subsidy from MEXT, 2006–2011, and the Global Center of Excellence Program from MEXT, 2007–2012.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Graham Wallace.
Rights and permissions
About this article
Cite this article
Takeiri, M., Tachibana, M., Kaneda, A. et al. Inhibition of macrophage activation and suppression of graft rejection by DTCM-glutarimide, a novel piperidine derived from the antibiotic 9-methylstreptimidone. Inflamm. Res. 60, 879–888 (2011). https://doi.org/10.1007/s00011-011-0348-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0348-z