Abstract
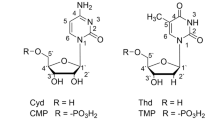
The relationship between penta-coordinate phosphorus compounds and biochemistry is briefly reviewed. Some interesting phenomena such as peptide formation, ester formation, ester exchange on phosphorus and N to O migration occur at room temperature when the amino group of amino acid is associated with phosphoryl group. Serine or threonine in conjugate of nucleo-side-amino acid could recognize different nucleobases. N-phosphoryl Histine and Ser-His dipeptide could cleavage nucleic acid, protein and ester in neutral medium. It is found that the above phenomena all undergo penta-coordinate intermediate of phosphorus atom, which is proposed as the key factor to determine their activities.
Similar content being viewed by others
References
Joyce, G. F., RNA evolution and origins of life, Nature, 1989, 338: 217–224.
Dickerson, R. E., Chemical evolution and the origin of life, Sci. Amer., 1978, 239(3): 70–86.
Orgel, L. E., The origin of life on the Earth, Sci. Amer., 1994, 271(4): 76–83.
Westheimer, F. H., Why nature chose phosphates, Science, 1987, 235(4793): 1173–1178.
Todd, L., Where there’s life, there’s phosphorus, in Science and Scientists (eds. Makoto, K., Keiko, N., Tairo, O.), Tokyo: Japan Sci. Soc. Press, 1981, 275–279.
Weckwerth, G., Schidlowski, M., Phosphorus as a potential guide in the search for extinct life on Mars, Adv. Space Res., 1995, 15(3): 185–191.
Nelson, L. D., Cox, M. M., Lehninger Principles of Biochemistry, 2nd ed., New York: Worth Publishers, Inc., 1993, 652–658.
van Ool, P. J. J. M., Buck, H. M., A quantum-chemical model description for the function of cyclic AMP, Its degradation and activation of protein kinase, Recl. Tranv. Chim. Pays-Bas, 1981, 100: 79–80.
van Ool, P. J. J. M., Buck, H. M., The mechanisms of action of cAMP: A quantum chemical study, Eur. J. Biochem., 1981, 121(2): 329–334.
Blatter, W. A., Knowles, J. R., Stereochemical course of glycerol kinase, pyruvate kinase, and hexokinase: phosphoryl transfer from chiral[γ(s)-16 O,17 O,18O]ATP, J. Am. Chem. Soc., 1979, 101: 510–511.
Kin, E. E., Wyckoff, H. W., Reaction mechanism of alkaline phosphatase based on crystal structures, J. Mol. Biol., 1991, 218: 449–464.
Anslyn, E., Breslow, R., On the mechanism of catalysis by ribonuclease: cleavage and isomerization of the dinucleotide UpU catalyzed by imidazole buffers, J. Am. Chem. Soc., 1989, 111(12): 4473–4482.
McGuigan, G., Cahard, D., Sheeka, H. M. et al., Aryl phosphoramidate derivatives of d4T have improved anti-HIV efficiency in tissue culture and may act by the generation of a novel intercellular metabolite, J. Med. Chem., 1996, 39(8): 1748–1753.
Wagner, C. R., McIntee, E. J., Schinazi, R. F. et al., Aromatic amino-acid phosphoramidate diesters and trimesters of 3′-azido-3′-deoxythymidine(AZT) are nontoxic inhibitors of HIV-1 replication, Bioorg. Med. Chem. Lett., 1995, 5(16): 1819–1824.
Balzarini, J., Egberink, H., Hartmann, K. et al., Antiretrovirus specificity and intracellular metabolism of 2′,3′-didehydro- 2′,3′-dideoxythymidine (stavudine) and its 5′-monophosphate triester prodrug So324, Mol. Pharmacol., 1996, 50(5): 1207–1213.
Valette, G., Pompon, A., Girardet, J. L. et al., Decomposition pathways andin vitro HIV inhibitory effects of IsoddA pronucleotides: Toward a rational approach for intracellular delivery of nucleoside 5′-monophosphates, J. Med. Chem., 1996, 39(10): 1981–1990.
Fu, H., Zhao, Y. F., Penta-coordinate phosphorus compounds of amino acid and nucleoside, Acta Chimica Sincia (in Chinese), 2000, 58(1): 6–12.
Zhang, B. Z., Zhang, G. T., Zhao, Y. F., The reactiviy study of N-(O,O-dialkyl)phosphoryl alanine with alcohol, Journal of Chinese Science and Technology University (in Chinese), 1994, 24: 496–499.
Li, Y. M., Yin, Y. W., Zhao, Y. F., Phosphoryl group participation leads to peptide formation from N-phosphorylamino acids, Int. J. Peptide Protein Res., 1992, 39: 375–381.
Ma, X. B., Zhao, Y. F., Phosphoryl group participation in the reactions of N-phosphoryldipeptide acids, Phosphorus, Sulfur and Silicon, 1992, 66: 107–114.
Li, Y. C., Tan, B., Zhao, Y. F., Phosphoryl transfer reaction of phosphohistidine, Heteroatom Chemistry, 1993, 4(4): 415–419.
Ma, X. B., Zhao, Y. F., Synthesis and novel properties of N-phosphoryl peptides, J. Org. Chem., 1989, 54(16): 4005–4008.
Yang, H. J., Liu, L., Liu, C. Y. et al., N-S phosphoryl migration in phosphoryl glutation, Int. J. Peptide Protein Res., 1993, 42: 39–43.
Zhao, Y. F., Yan, Q. J., Wang, Q. et al., Phosphoryl transfer reaction regulation by amino acid side chains: a model for phosphoproteins, Int. J. Peptide Protein Res., 1996, 46: 276–281.
Xue, C. B., Yin, Y. W., Zhao, Y. F., Studies on phosphoserine and phosphothreonine derivatives, Tetrahedron Lett., 1988, 29: 1145–1148.
Zhou, W. H., Ju, Y., Zhao, Y. F. et al., Simultaneous formation of peptides and nucleotides from N-phosphothreonine, Origins Life Evol. B, 1996, 26(6): 547–560.
Fu, H., Li, Z. L., Zhao, F. F. et al., Oligomerization of N, O-bis(trimethylsilyl)-alpha-amino acids into peptides mediated byo-phenylene phosphorochloridate, J. Am. Chem. Soc., 1999, 121(2): 291–295.
Chen, X., Zhao, Y. F., Ester exchange reaction of oxyphosphorane with thymidine, Synthetic Commun., 1995, 25(22): 3691–3694.
Li, Y. S., Zhao, Y. F., Hatfield, S. et al., Dipeptide seryl-histidine and related oligopeptides cleave DNA, protein, and a carboxyl ester, Bioorg. Med. Chem., 2000, 8(12): 2675–2680.
Li, Y. F., Sha, Y. W., Ma, Y. et al., Cleavage of DNA by N-phosphoryl histidine, Biochem. Biophy. Res. Commun., 1995, 213(3): 875–880.
Zhao, Y. F., Cao, P. S., Phosphoryl amino-acids common origin for nucleic-acids and protein, J. Biol. Phys., 1994, 20: 283–287.
Zhou, W. H., Ju Y., Zhao, Y. F. et al., Stimultaneous formation of peptides from N-phosphothreonine, Origins Life Evol. B, 1996, 26: 547–556.
Zhao, Y. F., Cao, P. S., Why nature chose alpha-amino acids, Pure Appl. Chem., 1999, 71(6): 1163–1166.
Landweber, L. F., Testing ancient RNA-protein interactions, Proc. Natl. Acad. Sci. USA, 1999, 96(20): 11067–11068.
Holmes, R. R., Hexacoordinate phosphorus via donor interaction, Implications regarding enzymatic reaction intermediates, Acc. Chem. Res., 1998, 31(9): 535–542.
Messmore, J. M., Raines, R. T., Pentavalent organo-vanadates as transition state analogues for phosphoryl transfer reactions, J. Am. Chem. Soc., 2000, 122(41): 9911–9916.
Parang, K., Till, J. H., Ablooglu, A. J. et al., Mechanism-based design of a protein kinase inhibitor, Nat. Struct. Biol., 2001, 8(1): 37–41.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, C., Li, Y., Cheng, C. et al. Penta-coordinate phosphorous compounds and biochemistry. Sc. China Ser. B-Chem. 45, 337–348 (2002). https://doi.org/10.1007/BF02879344
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02879344