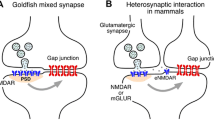
Abstract
In the develo** mammalian, neocortex gap junctions represent a transient, metabolic, and electrical communication system. These gap junctions may play a crucial role during the formation and refinement of neocortical synaptic circuitries. This article focuses on two major points. First, the influence of gap junctions on electrotonic cell properties will be considered. Both the time-course and the amplitude of synaptic potentials depend,inter alia, on the integration capabilities of the postsynaptic neurons. These capabilities are, to a considerable extent, determined by the electrotonic characteristics of the postsynaptic cell. As a consequence, the efficacy of chemical synaptic inputs may be crucially affected by the presence of gap junctions.
The second major topic is the regulation of gap junctional communication by neurotransmitters via second messenger pathways. The monoaminergic neuromodulators dopamine, nordrenaline, and serotonin reduce gap junction coupling via activation of two different intracellular signaling cascades—the cAMP/protein kinase A pathway and the IP3/Ca2+/protein kinase C pathway, 013 respectively. In addition, gap junctional communication seems to be modulated by the nitric oxide (NO)/cGMP system. Since NO production can be stimulated by glutamate-induced calcium influx, the NO/cGMP-dependent modulation of gap junctions might represent a functional link between develo** glutamatergic synaptic transmission and the gap junctional network. Thus, it might be of particular importance in view of a role of gap junctions during the process of circuit formation.
Similar content being viewed by others
References
Bayer E. C. (1993) Gap junctions.Int. Rev. Cytol. 137C, 1–37.
Yamada E. and Ishikawa T. (1965) Fine structure of the horizontal cells in some vertebrate retinae.Cold Spring Harbour Symp. Quant. Biol. 30, 383–392.
Naka K. I. and Rushton W. A. H. (1967) The generation and spread of S-potentials in fish (Cyprinidae).J. Physiol. 192, 437–461.
Witkovsky P. and Dowling J. E. (1969) Synaptic relationship in the plexiform layers of the carp retina.Z. Zellforsch. Mikrosk. Anat. 100, 60–82.
Kaneko A. (1971) Electrical connections between horizontal cells in the dogfish retina.J. Physiol. 213, 95–105.
Perlman I. and Ammermüller J. (1994) Receptive-field size of L1 horizontal cells in the turtle retina: effects of dopamine and background light.J. Neurophysiol. 72, 2786–2795.
Furukawa T. and Furshpan E. J. (1963) Two inhibitory mechanisms in the Mauthner neurones of the goldfish.J. Neurophysiol. 26, 140–176.
Lin J.-W. and Faber D. S. (1988) Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. I. Characteristics of electrotonic and chemical postsynaptic potentials.J. Neurosci. 8, 1302–1312.
Llinas R., Baker R., and Sotelo C. (1974) Electrotonic coupling between neurones in cat inferior olive.J. Neurophysiol. 37, 560–571.
Sotelo C., Llinas R., and Baker R. (1974) Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling.J. Neurophysiol. 37, 541–559.
Llinas R. and Yarom Y. (1986) Oscillatory properties of guinea pig inferior olivary neurones and their pharmacological modulation: an in vitro study.J. Physiol. (Lond.) 376, 163–182.
Baker R. and Llinas R. (1971) Electrotonic coupling between neurons in the rat mesencephalic nucleus.J. Physiol. Lond. 212, 45–63.
Korn H., Sotelo C., and Crepel F. (1973) Electrotonic coupling between neurones in the rat lateral vestibular nucleus.Exp. Brain Res. 164, 255–275.
Andrew R. D., MacVicar B. A., Dudek F. E., and Hatton G. I. (1981) Dye transfer through gap junctions between neuroendocrine cells of rat hypothalamus.Science 211, 1187–1189.
O’Donnell P. and Grace A. A. (1993) Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens.J. Neurosci. 13, 3456–3471.
Macvicar B. A. and Dudek F. E. (1981) Electrotonic coupling between pyramidal cells: a direct demonstration in rat hippocampal slices.Science 213, 782–785.
Perez-Velaquez J. L., Valliante T. A., and Carlen P. L. (1994) Modulation of gap junctional mechanisms during calcium-free induced field burst activity: a possible role for electrotonic coupling in epileptogenesis.J. Neurosci. 14, 4308–4317.
Connors B. W., Benardo L. S., and Prince D. A. (1983) Coupling between neurons of the develo** rat neocortex.J. Neurosci. 3, 773–782.
Peinado A., Yuste R., and Katz L. C. (1993) Extensive dye coupling between rat neocortical neurons during the period of circuit formation.Neuron 10, 103–114.
Spitzer N. C. (1982) Voltage- and stage-dependent uncoupling of Rohon-Beard neurones during embryonic development of Xenopus tadpoles.J. Physiol. (Lond.) 330, 145–162.
Walton K. D. and Navarrete R. (1991) Postnatal changes in motoneurone electrotonic coupling studied in the in vitro rat lumbar spinal cord.J. Physiol. (Lond.) 433, 283–305.
Fulton B. P., Miledi R., and Takahashi T. (1980) Electrical synapses between motorneurons in the spinal cord of the newborn rat.Proc. R. Soc. Lond. (Biol.) 208, 115–120.
Paternostro M. A., Reyher C. K., and Brunjes P. C. (1995) Intracellular injections of lucifer yellow into lightly fixed mitral cells reveal neuronal dye-coupling in the develo** rat olfactory bulb.Dev. Brain Res. 84, 1–10.
Lo Turco J. J. and Kriegstein A. R. (1991) Clusters of coupled neuroblasts in embryonic neocortex.Science 252, 563–566.
Lo Turco J. J. and Kriegstein A. R. (1995) Neurotransmitter signaling before the birth of neurones, inThe Cortical Neuron (Gutnick M. D. and Mody I., eds.) Oxford University Press, New York, pp. 197–209.
Mienville J. M., Lange G. D., and Barker J. L. (1994) Reciprocal expression of cell-cell coupling and voltage-dependent Na current during embryogenesis of rat telencephalon.Dev. Brain Res. 77, 89–95.
Rörig B., Klausa G., and Sutor B. (1996) Intracellular acidification reduced gap junction coupling between immature rat neocortical pyramidal neurones.J. Physiol. 490, 31–49.
Dermietzel R. O., Traub T., Hwang K., Beyer E., Bennett M. V. L., Spray D. C., and Willecke K. (1989) Differential expression of three gap junction proteins in develo** and mature brain tissue.Proc. Natl. Acad. Sci. USA 86, 10,148–10,152.
Rörig B., Klausa G., and Sutor B. (1995) Dyecoupling between pyramidal neurons in develo** rat prefrontal and frontal cortex is reduced by protein kinase A activation and dopamine.J. Neurosci. 15, 7386–7400.
Fulton B. P. (1994) Gap junctions in the develo** nervous system.Perspect. Dev. Neurobiol. 2, 327–334.
Nadarajah B. and Parnavelas J. G. (1995) Gap junctions in the develo** rat cerebral cortex.Soc. Neurosci. Abstracts 21, 29.
Penttonen M., Bragin A., Sik A., and Buszaki G. (1995) Dye-coupling of neurones in the hippocampus implies a role for gap junctions in epilepsy.Soc. Neurosci. Abstracts 21, 1971.
Saez J. C., Berthoud V. M., Moreno A. P., and Spray D. C. (1993) Gap junctions. Multiplicity of controls in differentiated and undifferentiated cells and possible functional implications.Adv. Second Mess. Phosphoprotein Res. 27, 163–198.
Gutnick M. J. and Prince D. A. (1981) Dye coupling and possible electrotonic coupling in the guinea pig neocortical slice.Science 211, 67–70.
Schulte-Mattler M. and Luhmann H. J. (1995) Morphometrical analysis of dye-coupled cells in adult rat neocortex.Pflügers Arch. (Suppl.) 429, R27.
Yuste R., Peinado A., and Katz L. C. (1992) A system of neuronal domains in develo** neocortex.Science 257, 665–668.
Yuste R., Nelson D. A., Rubin W. W., and Katz L. C. (1995) Neuronal domains in develo** neocortex: mechanisms of coactivation.Neuron 14, 7–17.
Kandler K. and Katz L. C. (1995) Mechanisms for initiation and propagation of intercellular calcium waves in the develo** visual cortex.Soc. Neurosci. Abstracts 21, 1285.
Perkins A. T. and Teyler T. J. (1988) A critical period for long-term potentiation in the develo** rat visual cortex.Brain Res. 439, 222–229.
Katz L. C. (1995) Coordination of vertebrate cellular assemblies by gap junctions.Dev. Biol. 6, 117–125.
Kandler K. and Katz L. C. (1995) Neuronal coupling and uncoupling in the develo** nervous system.Curr. Opin. Neurobiol. 5, 98–105.
Rörig B., Klausa G., and Sutor B. (1995) Beta-adrenoreceptor activation reduces dye-coupling between immature rat neocortical neurones.Neuro Report 6, 1811–1815.
Rörig B. and Sutor B. (1996) Nitric oxide stimulated increase in intracellular cGMP modulates gap junction coupling in rat neocortex.Neuro Report 7, 569–572.
Roerig B. and Sutor B. (1996) Serotonin regulates gap junction coupling in the develo** rat somatosensory cortex.Eur. J. Neurosci. 8, in press.
Chesler M. and Kaila K. (1992) Modulation of pH by neuronal activity.TINS 15, 396–402.
Hablitz J. J. (1987) Spontaneous ictal-like discharges and sustained potential shifts in the develo** rat neocortexJ. Neurophysiol. 58, 1052–1065.
Sutor B., Hablitz J. J., Rucker F., and ten Bruggencate G. (1994) Spread of epileptiform activity in the immature rat neocortex studied with voltage-sensitive dyes and laser scanning microscopy.J. Neurophysiol. 72, 1756–1768.
Connors B. W., Benardo L. S., and Prince D. A. (1984) Carbon dioxide sensitivity of dye coupling among glia and neurons of the neocortex.J. Neurosci. 4, 1324–1330.
Bigiani A. and Roper S. D. (1994) Reduction of electrical coupling between Necturus taste receptor cells a possible role in acid taste.Neurosci. Lett. 176, 212–216.
Miyachi E. I., Kato C., and Nakaki T. (1994) Arachidonic acid blocks gap junctions between retinal horizontal cells.NeuroReport 5, 485–488.
Rall W. (1969) Time constant and electrotonic length of membrane cylinders and neurons.Biophys. J. 9, 1483–1508.
Johnston D. and Brown T. H. (1983) Interpretation of voltage-clamp measurements in hippocampal neurons.J. Neurophysiol. 50, 464–486.
Spruston N., Jaffe D. B., Williams S. H., and Johnston D. (1993) Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events.J. Neurophysiol. 70, 781–802.
Harris A. L., Spray D. C., and Bennett M. V. L. (1981) Kinetic properties of a voltage-dependent junctional conductance.J. Gen. Physiol. 77, 95–117.
Barrio L. C., Suchyna T., Bargiello T. A., **an Hu I., Rogers R., Bennett M. V. L., and Nicholson B. (1991) Gap junctions formed by connexin 26 and 32 alone and in combination are differently affected by applied voltage.Proc. Natl. Acad. Sci. USA 88, 8410–8414.
Bennett M. V. L., Rubin J. B., Bargiello T. A., and Verselis V. K. (1993) Structure-function studies of voltage sensitivity of connexins, the family of gap junction forming proteins.Jpn. J. Physiol. 43, 301–310.
Verselis V. K., Ginter C. S., and Bargiello T. A. (1994) Opponent voltage gating polarities of two closely related connexins.Nature (Lond.) 368, 348–351.
White T. W., Bruzzone R., Goodenough D. A., and Paul G. K. (1994) Voltage gating of connexins.Nature (Lond.) 371, 208–209.
Bukauskas F. F. and Weingart R. (1994) Voltage-dependent gating of single gap junction channels in an insect cell line.Biophys. J. 67, 613–625.
Bukauskas F. F., Elfgang C., Willecke K., and Weingart R. (1995) Heterotypic gap junction channels (connexin26-connexin32) violate the paradigm of unitary conductance.Pflügers Arch. 429, 870–872.
Meyer T. (1991) Cell signalling by second messenger waves.Cell 64, 675–678.
Paysan J., Bolz J., Mohler H., and Fritschy J.-M. (1994) GABAA receptor α1 subunit, an early marker for area specification in develo** rat cerebral cortex.J. Comp. Neurol. 350, 133–149.
Piccolino M., Neyton J., and Gerschenfeld H. M. (1984) Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3′,5′-mono-phosphate in horizontal cells of the turtle retina.J. Neurosci. 4, 2477–2488.
Lasater E. M. and Dowling J. E. (1985) Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells.Proc. Natl. Acad. Sci. USA 82, 3025–3029.
De Vries S. H. and Schwartz E. A. (1992) Hemi-gap-junction channels in solitary horizontal cells of the catfish retina.J. Physiol. 445, 201–230.
Hampson E. C. G. M., Vaney D. I., and Weiler R. (1992) Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina.J. Neurosci. 12, 4911–4922.
McMahon D. G. and Brown D. R. (1994) Modulation of gap-junction channel gating at zebrafish retinal electrical synapses.J. Neurophysiol. 72, 2257–2259.
Cepeda J., Walsh J. P., Hull C. D., Howard S. G., Buchwald N. A., and Levine M. S. (1989) Dye coupling in the striatum of the rat. I. Modulation by dopamine-depleting lesions.Synapse 4, 229–237.
Onn S.-P. and Grace A. A. (1994) Dye coupling between rat striatal neurons recorded in vivo: compartmental organization and modulation by dopamine.J. Neurophysiol. 71, 1917–1934.
Pereda A., Triller A., Wolzon L., and Faber D. S. (1994) Postsynaptic modulation of synaptic efficacy at mixed synapses on the Mauthner cell.J. Neurosci. 14, 3704–3712.
Saez J. C., Berthood V. M., Kadle R., Traub O., Nicholson B. J., Bennett M. V. L., and Dermietzel R. (1991) Pinealocytes in rats: connexin identification and increase in coupling caused by norepinephrine.Brain Res. 568, 265–275.
Giaume C., Marin P., Cordier J., Glowinski J., and Premont J. (1991) Adrenergic regulation of intercellular communication between striatal astrocytes from the mouse.Proc. Natl. Acad. Sci. USA 88, 5577–5581.
Haydon P. G., McCobb D. P., and Kater S. B. (1984) Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons.Science 226, 561–564.
Piccolino M., Neyton J., Witkovsky P., and Gerschenfeld H. M. (1982) Gamma-aminobutyric acid antagonists decrease junctional communication between L-horizontal cells of the retina.Proc. Natl. Acad. Sci. USA 79, 3671–3675.
Iwatsuki N. and Peterson O. H. (1978) Pancreatic acinar cells: acetylcholine-evoked electrical uncoupling and its ionic dependency.J. Physiol. 274, 81–86.
Hatton G. I. and Yang Q. Z. (1990) Activation of excitatory amino acid inputs to supraoptic neurones. I. Induced increases in dye-coupling in lactating, but not virgin or male rats.Brain Res. 513, 264–269.
Hatton G. I. and Yang Q. Z. (1996) Synaptically released histamine increases dye coupling among vasopressinergic neurones of the supraoptic nucleus: mediation by H1 receptors and cyclic nucleotides.J. Neurosci. 16, 123–129.
Hatton G. I., Yang Q. Z., and Koran L. E. (1992) Effects of ovariectomy and estrogen replacement on dye coupling among rat supraoptic nucleus neurones.Brain Res. 572, 291–295.
Schmidt R. H., Björklund A., Lindvall O., and Loren I. (1982) Prefrontal cortex: dense dopaminergic input in the newborn rat.Dev. Brain Res 5, 222–228.
Verney C., Berger G., Adrian J., Vigny A., and Gay M. (1982) Development of the dopaminergic innervation of the rat cerebral cortex: a light microscopic immunocytochemical study using anti-tyrosine hydroxylase antibodies.Dev. Brain Res. 5, 41–52.
Berger B., Verney C., Alvarez C., Vigny A., and Helle K. B. (1985) New dopaminergic terminal fields in the motor, visual (area 18b) and retrosplenial cortex in the young and adult rat. Immunocytochemical and catecholamine histochemical analysis.Neuroscience 15, 983–998.
Berger B., Gaspar P., and Verney C. (1991) Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates.TINS 14, 21–27.
Kalsbeek A., Voorn P., Buijs R. M., Pool L. W., and Uylings H. B. M. (1988) Development of the dopaminergic innervation in the prefrontal cortex of the rat.J. Comp. Neurol. 269, 58–62.
Schambra U. B., Duncan G. E., Breese G. R., Fornaretto M. G., Caron M. G., and Fremeau R. T. (1994) Ontogeny of D1A and D2 receptor subtypes in rat brain using in situ hybridization and receptor binding.Neuroscience 62, 65–85.
Kalsbeek A., Buijs R. M., Hofman M. A., Matthijsen M. A. H., Pool C. W., and Uylings H. B. M. (1987) Effects of neonatal thermal lesioning of the mesocortical dopaminergic projection on the development of the rat prefrontal cortex.Dev. Brain. Res. 32, 123–132.
Leslie C. A., Robertson M. W., Cutler A. J., and Bennett J. P. (1991) Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: a quantitative autoradiographic analysis.Dev. Brain Res. 62, 109–114.
Kalsbeek A., Matthissen M. A. H., and Uylings H. B. M. (1989) Morphometric analysis of prefrontal cortical development following neonatal lesioning of the dopaminergic mesocortical projections.Exp. Brain Res. 78, 279–289.
Saez J. C., Spray D. C., Nairn A. C., Hertzberg E. L., Greengard P., and Bennett M. V. L. (1986) CAMP increases junctional conductance and stimulates phosphorylation of the 27 kDa principal gap junction polypeptide.Proc. Natl. Acad. Sci. USA 83, 2473–2477.
DeMello W. C. (1983) The influence of pH on the healing-over of mammalian cardiac muscle.J. Physiol. 339, 299–307.
DeMello W. C. (1989) Effect of isoproterenol and 3-isobutyl-1-methylxanthine on junctional conductance in heart cell pairs.Biochim. Biophys. Acta 1012, 291–298.
Burt J. M. and Spray D. C. (1988) Single channel events and gating behaviour of the cardiac gap junction channel.Proc. Natl. Acad. Sci. USA 85, 3431–3434.
Burt J. M. and Spray D. C. (1988) Inotropic agents modulate gap junctional conductance between cardiac myocytes.Am. J. Physiol. 254, 1206–1210.
Teranishi T., Negishi K., and Kato S. (1983) Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina.Nature 301, 243–246.
Lasater E. M. (1987) Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic-AMP-dependent protein kinase.Proc. Natl. Acad. Sci. USA 84, 7319–7323.
McMahon D. G. (1994) Modulation of electrical synaptic transmission in zebrafish retinal horizontal cells.J. Neurosci. 14, 1722–1734.
LaHoste G. J., Yu J., and Marshall J. F. (1993) Striatal fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity.Proc. Natl. Acad. Sci. USA 90, 7451–7455.
Robertson G. S. and Robertson H. A. (1987) D1 and D2 dopamine agonist synergism: separate sites of action?TIPS 8, 295–299.
Saller C. F. and Salama A. I. (1986) D1- and D-2 Dopamine receptor blockade: interactive effects in vitro and in vivo.J. Pharmacol. Exp. Ther. 236, 714–720.
Maeda T., Tohyama M., and Shimizu N. (1974) Modification of postnatal development of neocortex in rat brain with experimental deprivation of locus coeruleus.Brain Res. 70, 515–520.
Wendlandt S., Crow T. J., and Stirling R. V. (1977) The involvement of the noradrenergic system arising from the locus coeruleus in the postnatal development of the cortex of the rat brain.Brain Res. 125, 1–9.
Felten D. L., Hallman H., and Jonsson G. (1982) Evidence for a neurotrophic role of noradrenaline neurons in the postnatal development of rat cerebral cortex.J. Neurocytol. 11, 119–135.
Parnavelas J. G. and Blue M. E. (1982) The role of the noradrenergic system on the formation of synapses in the visual cortex of the rat.Dev. Brain Res. 3, 140–144.
Osterheld-Haas M. C., Van der Loos H., and Hornung J.-P. (1994) Monoaminergic afferents to cortex modulate structural plasticity in the barrelfield of the mouse.Dev. Brain Res. 77, 189–202.
Gordon B., Allen E. E., and Trombley P. Q. (1988) The role of norepinephrine in plasticity of visual cortex.Prog. Neurobiol. 30, 171–191.
Bröcher S., Artola A., and Singer W. (1992) Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex.Brain Res. 573, 27–36.
Levitt P. and Moore R. Y. (1979) Development of the noradrenergic innervation of neocortex.Brain Res. 162, 243–259.
McDonald J. K., Petrovic S. L., McCann S. M., and Parnavelas J. G. (1982) The development of beta-adrenergic receptors in the visual cortex of the rat.Neuroscience 7, 2649–2655.
Rhoades R. W., Bennett-Clarke C. A., Chiaia N. L., White F. A., McDonald G. J., Haring J. H., and Jaquin M. F. (1990) Development and lesion-induced reorganization of the cortical representation of the rat’s body surface as revealed by immunocytochemistry for serotonin.J. Comp. Neurol. 293, 190–207.
D’Amato R. J., Blue M. E., Largent B. L., Lynch D. R., Ledbetter D. J., Molliver M. E., and Snyder S. H. (1987) Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas.Proc. Natl. Acad. Sci. USA 84, 4322–4326.
Nakazawa M., Koh T., Kani K., and Maeda T. (1992) Transient patterns of serotonergic innervation in the rat visual cortex: normal development and effects of neonatal enucleation.Dev. Brain Res. 66, 77–90.
Hohmann C. F., Hamon R., Batshaw M. L., and Coyle J. T. (1988) Transient postnatal elevation of serotonin levels in mouse neocortex.Dev. Brain Res. 43, 163–166.
Killackey H. P. and Leshin S. (1975) The organization of specific thalamocortical projections to the posteromedial barrel subfield of the rat somatic sensory cortex.Brain Res. 86, 469–472.
Lidov H. G. W. and Molliver M. E. (1982) An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields.Brain Res. Bull. 8, 389–430.
Fujimiya M., Kimura H., and Maeda T. (1986) Postnatal development of serotonin nerve fibers in the somatosensory cortex of mice studied by immunocytochemistry.J. Comp. Neurol. 246, 191–201.
Bennett-Clarke C. A., Hankin M. H., Leslie M. J., Chiaia N. L., and Rhoades R. W. (1994) Patterning of the neocortical projections from the raphe nuclei in perinatal rats: investigation of potential organizational mechanisms.J. Comp. Neurol. 348, 277–290.
Bennett-Clarke C. A., Leslie M. J., Lane R. D., and Rhoades R. W. (1994) Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat’s somatosensory cortex.J. Neurosci. 14, 7595–7607.
Gu Q. and Singer W. (1995) Involvement of serotonin in developmental plasticity of kitten visual cortex.Eur. J. Neurosci. 7, 1146–1153.
Rhoades R. W., Bennett-Clarke C. A., Shi M.-Y., and Mooney R. D. (1994) Effects of 5-HT on thalamocortical synaptic transmission in the develo** rat.J. Neurophysiol. 72, 2438–2450.
Bennett-Clarke C. A., Leslie M. J., Chaia N. L., and Rhoades R. W. (1993) Serotonin 1 B receptors in the develo** somatosensory and visual cortices are located on thalamocortical axons.Proc. Natl. Acad. Sci. USA 90, 153–157.
Miller M. W. (1988) Development of projection and local circuit neurons in neocortex, inCerebral Cortex, vol. 7, Development and Maturation of Cerebral Cortex. (Peters A. and Jones E. D., eds.) Plenum, New York, pp. 133–175.
Rörig B., Klausa G., and Sutor B. (1995) Modulatory neurotransmitters reduce dyecoupling between develo** lamina II/III pyramidal neurones in rat neocortex.Soc. Neurosci. Abstracts 21, 1509.
Pazos A., Cortes R., and Palacios M. (1985) Quantitative autoradiographic map** of serotonin receptors in the rat brain. II. Serotonin-2 receptors.Brain Res. 346, 231–249.
Pompeiano M., Palacios J. M., and Mengod G. (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors.Mol. Brain Res. 23, 163–178.
Wright D. E., Seroogy K. B., Lundgren K. H., Davis B. M., and Jennes L. (1995) Comparative localization of serotonin 1A, 1 C and 2 receptor subtype mRNAs in rat brain.J. Comp. Neurol. 351, 357–373.
Pazos A. and Palacios J. M. (1985) Quantitative autoradiographic map** of serotonin receptors in the rat brain. I. Serotonin-1 receptors.Brain Res. 346, 205–230.
Pazos A., Cortes R., and Palacios M. (1985) Quantitative autoradiographic map** of serotonin receptors in the rat brain. II. Serotonin-2 receptors.Brain Res. 346, 231–249.
De Chaffoy de Courcelles D., Leysen J. E., de Clerk F., van Belle H., and Janssen P. A. J. (1985) Evidence that phospholipid turnover is the signal transducing system coupled to serotonin-S2 receptor sites.J. Biol. Chem. 260, 7603–7608.
Conn P. J., Sanders-Busch E., Hoffmann B. J., and Hartig P. R. (1986) A unique serotonin receptor in choroid plexus is linked to phosphatidylinositol turnover.Proc. Natl. Acad. Sci. USA 83, 4986–4088.
Noma A. and Tsuboi N. (1986) Direct measurement of the gap junctional conductance under the influence of Ca2+ in dissociated paired myocytes of guinea pig.Jpn. Heart J. 27, 161–166.
Lazrak A. and Peracchia C. (1993) Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells.Biophys. J. 65, 2002–2012.
Smith P. M. and Gallacher D. V. (1994) Thapsigargin-induced Ca2+ mobilization in acutely isolated mouse lacrimal acinar cells is dependent on a basal level of Ins(1,4,5)P3 and is inhibited by heparin.Biochem. J. 299, 37–40.
Takahashi M., Tanzawa K., and Takahashi S. (1994) Adenophostins, newly discovered metabolites ofPenicillium brevicompactum, act as potent agonists of the inositol 1,4,5-trisphosphate receptor.J. Biol. Chem. 269, 369–372.
Sullivan J. P., Connor J. R., Shearer B. G., and Burch R. M. (1991) ‘2,6-Diamino-N-([1-(oxotridecyl)-2-piperidinyl]methyl)hexanamide (NPC 15437): a selective inhibitor of protein kinase C’.Agents Actions 34, 138.
Wang H. Y. and Friedman E. (1990) Central 5-hydroxytryptamine receptor linked protein kinase C translocation: a functional postsynaptic signal transduction system.Mol. Pharmacol. 37, 75–79.
Ngezahayo A., Lang F., and Kolb H. A. (1995) Cholecystokinin-octapeptide affects the fluorescence signal of a single pancreatic acinar cell loaded with the acrylodan-labelled MARCKS peptide, a protein kinase C substrate.Pflügers Arch. 429, 805–808.
Inoguchi T., Ueda F., Umeda F., Yamashita T., and Nawata H. (1995) Inhibition of intercellular communication via gap junctions in cultured aortic endothelial cells by elevated glucose and phorbol ester.Biochem. Biophys. Res. Commun. 208, 492–497.
Konietzko U. and Müller C. M. (1994) Astrocytic dye-coupling in rat hippocampus: topography, developmental onset, and modulation by protein kinase C.Hippocampus 4, 297–306.
Munari-Silem Y., Audebet C., and Rousset B. (1991) Hormonal control of cell to cell communication: regulation by thyrotropin of the gap junction-mediated dye transfer between thyroid cells.Endocrinology 128, 3299–3309.
Nnamani C., Godwin A., Ducsay C. A., Longo L. D., and Fletcher W. H. (1994) Regulation of cell-cell communication mediated by connexin 43 in rabbit myometrial cells.Biol. Reprod. 50, 377–389.
Berthoud V. M., Ledbetter M. L., Hertzberg E. L., and Saez J. C. (1992) Connexin43 in MDCK cells: regulation by a tumor-promoting phorbol ester and Ca2+.Eur. J. Biol. 57, 40–50.
Reynhout J. K., Lampe P. D., and Johnson R. G. (1992) An activator of protein kinase C inhibits gap junction communication between cultured bovine lens cells.Exp. Cell Res. 198, 337–342.
Enkvist M. O. and McCarthy K. D. (1992) Activation of protein kinase C blocks astroglial gap junction communication and inhibits the spread of calcium waves.J. Neurochem. 59, 519–526.
Berthoud V. M., Rook M. B., Traub O., Hertzberg E. L., and Saez J. C. (1993) On the mechanisms of cell uncoupling induced by a tumor phorbol ester in clone 9 cells, a rat liver epithelial cell line.Eur. J. Cell Biol. 62, 384–396.
Randriamampita C., Giaume C., Neyton J., and Trautmann A. (1988) Acetylcholine-induced closure of gap junction channels in rat lacrimal glands is probably mediated by protein kinase C.Pflügers Arch. 412, 462–468.
Saez J. M., Nairn A. C., Czernik A. J., Spray D. C., Hertzberg E. L., Greengard P., and Bennett M. V. (1990) Phosphorylation of connexin 32, a hepatocyte gap junction protein, by cAMP dependent protein kinase, protein kinase C and Ca2+/calmodulin dependent protein kinase II.Eur. J. Biochem. 192, 263–273.
Spray D. C. and Burt J. M. (1990) Structure-activity relations of the cardiac gap junction channel.Am. J. Physiol. 258, 195–205.
Rose B. and Loewenstein W. R. (1975) Permeability of cell junction depends on local cytoplasmic calcium activity.Nature 254, 250–252.
Noma A. and Tsuboi N. (1987) Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea pig.J. Physiol. Lond. 382, 193–211.
Crow J. M., Atkinson M. M., and Johnson R. G. (1994) Micromolar levels of intracellular calcium reduce gap junctional permeability in lens cultures.Invest. Opthalmol. Vis., Sci. 35, 3332–3341.
Rao G., Barnes C. A., and McNaughton B. L. (1987) Occlusion of hippocampal electrical junctions by intracellular calcium injection.Brain Res. 408, 267–270.
Kasai H. and Petersen O. H. (1994) Spatial dynamics of second messengers: IP3 and cAMP as long-range and associative messengers.TINS 17, 95–101.
Kandler K., Douglas S. B., and Katz L. C. (1994) Neuronal dye-coupling and spontaneous activity are inversely related in develo** ferret visual cortex.Soc. Neurosci. Abstracts 20, 215.
Burgard E. C. and Hablitz J. J. (1993) Developmental changes in NMDA and non-NMDA receptor-mediated synaptic potentials in rat neocortex.J. Neurophysiol. 69, 230–240.
Vincent S. R. (1993) Nitric oxide: a radical neurotransmitter in the central nervous system.Prog. Neurobiol. 42, 129–160.
Crair M. C. and Malenka R. C. (1995) A critical period for long-term potentiation at thalamocortical synapses.Nature 375, 325–328.
Carmignoto G. and Vicini S. (1992) Activity dependent decrease in NMDA receptor responses during development of the visual cortex.Science 258, 1007–1011.
Jonas P., Racca C., and Sakmann B. (1994) Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurones caused by differential GluR-B subunit expression.Neuron 12, 1281–1289.
Miyachi E.-I., Murakami M., and Nakaki T. (1990) Arginine blocks gap junctions between retinal horizontal cells.NeuroReport 1, 107–110.
Kwak B. R., Saez J. C., Wilders R., Chanson M., Fishman G. I., Hertzberg E. L., Spray D. C., and Jongsma H. J. (1995) Effects of cGMP-dependent phosphorylation on rat and human connexin 43 gap junction channels.Pflügers Arch. 430, 770–778.
Alagarsamy S., Lonart C., and Johnson K. M. (1994) Regulation of nitric oxide synthase activity in cortical slices by excitatory amino acids and calcium.J. Neurosci. Res. 38, 648–653.
Price R. H., Mayer B., and Beitz A. J. (1993) Nitric oxide synthase neurones in rat brain express more NMDA receptor mRNA than non-NOS neurons.NeuroReport 4, 807–810.
Yan X. X., Garey L. J., and Jen L. S. (1994) Development of NADH-diaphorase activity in the rat neocortex.Dev. Brain Res. 79, 29–38.
Valtschanoff J. G., Weinberg R. J., Kharazia V. N., Schmidt H. H., Nakane M., and Rushioni, A. (1993) Neurons in rat cerebral cortex that synthesize nitric oxide: NADPH diaphorase histochemistry, NOS immunohistochemistry and colocalization with GABA.Neurosci. Lett. 157, 157–161.
Kitchener P. D., Van-der-Zee C. E., and Diamond J. (1993) Lesion-induced NADPH-diaphorase reactivity in neocortical pyramidal neurones.NeuroReport 4, 487–490.
Dehay C., Giroud P., Berland M., Smart I., and Kennedy H. (1993) Modulation of the cell cycle contributes to the parcellation of the primate visual cortex.Nature 366, 464–466.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rörig, B., Sutor, B. Regulation of gap junction coupling in the develo** neocortex. Mol Neurobiol 12, 225–249 (1996). https://doi.org/10.1007/BF02755590
Issue Date:
DOI: https://doi.org/10.1007/BF02755590