Summary
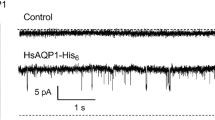
The major intrinsic protein (MIP26) of bovine lens membranes, purified by HPLC, was incorporated into liposomes and planar bilayers. Permeability of MIP26 channels was studied in liposomes by a spectrophotometric osmotic-swelling assay, and channel electrical properties were monitored in planar bilayers following liposome fusion. Particle formation in liposomes was determined by freeze fracture. MIP26 channels were permeable to KCl and sucrose. In planar bilayers, channel-conductance transitions were observed only after addition of liposomes to both chambers and with voltages greater than ±20 mV. Channel open probability decreased progressively as voltage increased, and an open probability of 50% was at 60–80 mV, indicating that the channels are voltage dependent. Histograms of single-channel current amplitudes at 80 mV showed a Gaussian distribution that peaked at 10 pA (∼120 pS), after subtraction of 1 pA baseline current. Frequency distributions of open and closed times at 80 mV were single exponential functions with time constants of 0.13 and 1.9 sec, respectively. Open time constants ranged from 0.1 to 0.3 sec, and closed time constants ranged from 1 to 7 sec. Cs+ did not decrease conductance, but reduced mean open time from 0.2 to 0.038 sec and mean closed time from 1.5 to 0.38 sec. The increase in channel flickering with Cs+ occurred in bursts. TEA affected neither conductance nor kinetics. Channel events were also observed in Na+ solutions (zero K+). These data indicate that MIP26 channels are not K+-selective channels. Channel characteristics such as: permeability to molecules larger than small ions, conductance greater than 100 pS, long open and closed time constants, etc., are similar to those of gap junction channels.
Similar content being viewed by others
References
Alvarez, O. 1986. How to set up a bilayer system.In: Ion Channel Reconstitution C. Miller, editor. pp. 115–130. Plenum New York
Benedetti, E.L., Dunia, I., Bentzel, C.J., Vermorken, A.J.M., Kibbelaar, M., Bloemendal, H. 1976. A portrait of plasma membrane specializations in eye lens epithelium and fibers.Biochim. Biophys. Acta,457:353–384
Bernardini, G., Peracchia, C. 1981. Gap junction crystallization in lens fibers after an increase in cell calcium.Invest. Ophthalmol. Vis. Sci. 21:291–299
Beyer, E.C., Paul, D.L., Goodenough, D.A. 1990. Connexin family of gap junction proteins.J. Membrane Biol. 116:187–194
Bok, D., Dockstader, J., Horwitz, J. 1982. Immunocytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions.J. Cell Biol. 92:213–220
Brewer, G.J. 1991. Reconstitution of lens channels between two membranes.In: Biophysics of Gap Junction Channels. C. Peracchia, editor. pp. 301–316. CRC Press, Boca Raton (FL)
Brewer, G.J., Dong, R.G. 1990. Transjunctional lens channels reconstituted: Regulation by Ca++ and calmodulin.J. Cell Biol. 111:65a
Brito, R.M.M., Vaz, L.C. 1986. Determination of the critical micelle concentration of surfactants using the fluorescent probeN-phenyl-1-naphthylamine.Anal. Biochem. 152:250–255
Colquhoun, D., Hawkes, A.G. 1983. The principles of the stochastic interpretation of ion-channel mechanisms.In: Single-Channel Recording. B. Sakmann and E. Neher, editors. pp. 135–175, Plenum, New York
Dunia, I., Manenti, S., Rousselet, A., Benedetti, E.L. 1987. Electron microscopic observations of reconstituted proteoliposomes with the purified major intrinsic membrane protein of eye lens fibers.J. Cell Biol. 105:1679–1689
Ehring, G.R., Hall, J.E. 1991. Reconstitution into planar lipid bilayers: Application to the study of lens MIP.In: Biophysics of Gap Junction Channels. C. Peracchia, editor. pp. 333–351. CRC Press, Boca Raton (FL)
Ehring, G.R., Zampighi, G., Horwitz, J., Bok, D., Hall, J.E. 1990. Properties of channel reconstituted from the major intrinsic protein of lens fiber membranes.J. Gen. Physiol. 96:631–664
Fitzgerald, P.G., Bok, D., Horwitz, J. 1983. Immunocytochemical localization of the main intrinsic polypeptide (MIP) in ultrathin frozen sections of rat lens.J. Cell Biol. 97:1491–1499
Gandolfi, S.A., Duncan, G., Tomlinson, J., Maraini, G. 1990. Mammalian lens inter-fiber resistance is modulated by calcium and calmodulin.Curr. Eye Res. 9:533–541
Girsch, S.J. 1988. Purification of lens gap junction protein by reverse-phase HPLC. p. 125. Proc. VIII Int. Congr. Eye Research, San Francisco
Girsch, S.J., Peracchia, C. 1985. Lens cell-to-cell channel protein: I. Self-assembly into liposomes and permeability regulation by calmodulin.J. Membrane Biol. 83:217–225
Girsch, S.J., Peracchia, C. 1991. Calmodulin interacts with a C-terminus peptide from the lens membrane protein MIP26.Curr. Eye Res. (in press)
Gooden, M.M., Rintoul, D.A., Takehana, M., Takemoto, L. 1985. Major intrinsic polypeptide (MIP26) from lens membrane: Reconstitution into vesicles and inhibition of channel forming activity by peptide antiserum.Biochem. Biophys. Res. Commun. 128:993–999
Gorin, M.B., Yancey, S.B., Cline, J., Revel, J.P., Horwitz, J. 1984. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning.Cell 39:49–59
Gruijters, W.T.M., Kistler, J., Bullivant, S., Goodenough, D.A. 1987. Immunologicalization of a MP7O in lens fiber 16–17 nm intercellular junctions.J. Cell Biol. 104:565–572
Harris, A.L. 1991. Connexin32 forms ion channels in single artificial membranes.In: Biophysics of Gap Junction Channels. C. Peracchia, editor. pp. 373–389. CRC Press Boca Raton, (FL)
Hertzberg, E.L., Gilula, N.B. 1981. Liver gap junctions and lens fiber junctions: Comparative analysis and calmodulin interaction.Cold Spring Harbor Symp. Quant. Biol. 46:639–645
Johnson, R.G., Klukas, K.A., Tze-Hong, L., Spray, D.C. 1988. Antibodies to MP26 are localized to lens junctions, alter intercellular permeability, and demonstrate increased expression during development.In: Gap Junctions. E.L. Hertzberg and R.G. Johnson, editors. pp. 81–98. Alan R. Liss, New York
Kistler, J., Bullivant, S. 1987. Protein processing in lens intercellular junctions: Cleavage of MP70 to MP38.Invest. Ophthalmol. Vis. Sci. 28:1687–1692
Kistler, J., Christie, D., Bullivant, S. 1988. Homologies between gap junction proteins in lens, heart and liver.Nature 331:721–723
Kistler, J., Kirkland, B., Bullivant, S. 1985. Identification of a 70,000-D protein in lens membrane junctional domains.J. Cell Biol. 101:28–35
Lasalde, J.A., Zuazaga, C. 1991. Cholesterol enrichment decreases the conductance of nicotinic acetylcholine receptor channels in tissue cultured chick muscle.Biophys. J. 59: 444a
Lea, J.A., Duncan, G. 1991. Lens cell communication—from the whole organ to single channels.In: Biophysics of Gap Junction Channels. C. Peracchia, editor. pp. 353–371, CRC Press, Boca Raton (FL)
Louis, C.F., Hogan, P., Visco, L., Strasburg, G. 1990. Identity of the calmodulin-binding proteins in bovine lens plasma membrane.Exp. Eye Res. 50:495–503
Luckey, M., Nikaido, H. 1980. Specificity of diffusion channels produced by λ phage receptor protein ofEscherichia coli.Proc. Natl. Acad. Sci. U.S.A. 77:167–171
Malewicz, B., Kumar, V.V., Johnson, R.G., Baumann, W.J. 1990. Lipids in gap junction assembly and function.Lipids 25:419–427
Mueller, P., Rudin, D., Tien, H.T., Wescott, W.C. 1962. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system.Nature 194:979–980
Nikaido, H., Rosenberg, E.Y. 1985. Functional reconstitution of lens gap junction proteins into proteoliposomes.J. Membrane Biol. 85:87–92
Paul, D.L., Goodenough, D.A. 1983. Preparation, characterization and localization of antisera against bovine MP26 an integral protein from the lens fiber plasma membrane.J. Cell Biol. 96:625–632
Peracchia, C. 1978. Calcium effects on gap junction structure and cell coupling.Nature 271:669–671
Peracchia, C. 1988. The calmodulin hypothesis for gap junction regulation six years laterIn: Gap Junctions. E.L. Hertzberg and R.G. Johnson, editors. pp. 267–282. Alan R. Liss, New York
Peracchia, S., Girsch, S.J. 1985. Is the cleavable C-terminal arm of cell-to-cell channel protein the channel gate?Biochem. Biophys. Res. Commun. 133:688–695
Peracchia, C., Girsch, S.J. 1989. Calmodulin site at the C-terminus of the putative lens gap junction protein MIP26.Lens Eye Toxic. Res. 6:613–621
Peracchia, C., Peracchia, L.L. 1980a. Gap junction dynamics: reversible effects of divalent cations.J. Cell Biol. 87:708–718
Peracchia, C., Peracchia, L.L. 1980b. Gap junction dynamics: Reversible effects of hydrogen ions.J. Cell Biol. 87:719–727
Sas, D.F., Sas, J., Johnson, K.R., Menko, A.S., Johnson, R.G. 1985. Junctions between lens fiber cells are labeled with a monoclonal antibody shown to be specific for MP26.J. Cell Biol. 100:216–225
Shen, L., Shrager, P., Girsch, S., Donaldson, P., Peracchia, C. 1991. Functional reconstitution of HPLC-purified lens channel protein MIP26 in liposomes and planar bilayers.Biophys J. 59:438a
Spray, D.C., Saez, J.C., Brosius, D., Bennett, M.V.L., Hertzberg E.L. 1986. Isolated liver gap junctions: Gating of transjunctional currents is similar to that in intact pairs of rat hepatocytes.Proc. Natl. Acad. Sci USA 83:5494–5497
Vallon, O., Dunia, I., Favard-Sereno, C., Hoebeke, J., Benedetti, E.L. 1985. MP26 in the bovine lens: A post-embedding immunocytochemical study.Biol. Cell 53:85–88
Van den Eijden-van Raaij, A.J.M., de Leeuw, A.L.M., Broekhuyse, R.M. 1985. Bovine lens calmodulin. Isolation, partial characterization and calcium-independent binding to lens membrane proteins.Curr. Eye Res. 4:905–912
Van Eldick, L.J., Hertzberg, E.L., Berdan, R.C., Gilula, N.B. 1985. Interaction of calmodulin and other calcium-modulated proteins with mammalian and arthropod junctional membrane proteins.Biochem. Biophys. Res Commun. 126:825–832
Welsh, M.J., Aster, J.C., Ireland, M., Alcala, J., Maisel, H. 1982. Calmodulin binds to chick lens gap junction protein in a calcium-independent manner.Science 216:642–644
Yellen, G. 1984. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells.J. Gen. Physiol. 84:157–186
Young, D.-L., Cohen, Z.A., Gilula, N.B. 1987. Functional assembly of gap junction conductance in lipid bilayers: Demonstration that the major 27 kDa protein forms the junctional channel.Cell 48:733–743
Zampighi, G., Hall, J.E., Ehring, G.R., Simon, S.A. 1989. The structural organization and protein composition of lens fiber junctions.J. Cell Biol. 108:2255–2275
Zampighi, G.A., Hall, J.E., Kreman, M. 1985. Purified lens junctional protein forms channels in planar lipid films.Proc. Natl. Acad. Sci USA 82:8468–8472
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shen, L., Shrager, P., Girsch, S.J. et al. Channel reconstitution in liposomes and planar bilayers with HPLC-purified MIP26 of bovine lens. J. Membrain Biol. 124, 21–32 (1991). https://doi.org/10.1007/BF01871361
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01871361