Conclusions
-
1.
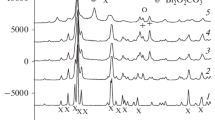
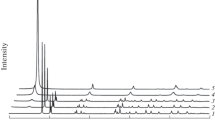
The mechanochemical activation of bayerite is accompanied by displacement of the hydroxyl units followed by splitting of the crystals into plates with thicknesses up to 10 å. During this displacement, the alternation of the units changes from ABAB... to ABBA..., which is characteristic of hydrargillite, and ABCABC..., which is characteristic of cubic packing.
-
2.
The activation process is also accompanied by dehydroxylation with the formation of O2− and molecular water, which is located predominantly in the first coordination sphere of Al(III) cations; the transformation of bayerite into a compound which is amorphous to x-rays and has the formula Al2O3·3H2O, octahedral coordination of the Al(III) cations, and disordered cubic packing of OH−, O2−, and H2O.
Similar content being viewed by others
Literature cited
B. P. Zolotovskii, O. P. Krivoruchko, T. A. Kriger, et al., Abstracts of Reports to the 8th All-Union Conference on the Kinetics and Mechanism of Solid-State Chemical Reactions [in Russian], Chernogolovka (1982), p. 286.
S. M. Paramzin, Yu. D. Pankrat'ev, E. A. Paukshtis, et al., Izv. Sibirsk. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, No. 11(4), 33 (1984).
S. M. Paramzin, O. P. Krivoruchko, B. P. Zolotovskii, et al., Izv. Sibirsk. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, No. 17(6), 39 (1984).
K. G. Rikhter, Methods for the Investigation of Catalytic Systems [in Russian], Nauka, Novosibirsk (1977), p. 5.
X-Ray PDF, JCPDS, Philadelphia, No. 20-11, 10–425.
V. M. Mastikhin, O. P. Krivoruchko, B. P. Zolotovskii, and R. A. Buyanov, React. Kinet. Catal. Lett.,18, Nos. 1–2, 117 (1981).
F. Zigan, W. Joswig, and N. Burger, Z. Kristallogr.,148, 255 (1978).
B. G. Linsen (ed.), Physical and Chemical Aspects of Adsorbents and Catalysts, Academic Press, London-New York (1970).
R. Rothbauer, F. Zigan, and H. O'Daniel, Z. Kristallogr.,125, 317–331 (1967).
A. Guinier, Théorie et Technique de la Radiocristallographie, 2nd ed., Dunod et Cie, Paris (1956).
R. E. Franklin, Acta Crystallogr.,4, 253 (1951).
B. E. Warren, Phys. Rev. Lett.,59, No. 9, 693 (1941).
B. G. érenburg, V. P. Fateeva, A. I. Min'kov, et al., Izv. Sibirsk. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, No. 12(5), 107 (1976).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 6, pp. 1209–1213, June, 1988.
Rights and permissions
About this article
Cite this article
Paramzin, S.M., Plyasova, L.M., Krivoruchko, O.P. et al. Investigation of the structural changes in bayerite during mechanochemical activation. Russ Chem Bull 37, 1061–1065 (1988). https://doi.org/10.1007/BF00961899
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00961899