Summary
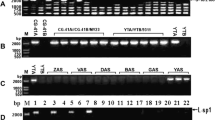
Fertile lines of sorghum (Sorghum bicolor) were shown to differ from cytoplasmic male sterile (CMS) lines by the presence of a 3.8 kb HindIII chloroplast DNA fragment in the former and a smaller (3.7 kb) fragment in the latter. DNA/DNA hybridization studies showed that these two fragments are homologous. Fertile plants from S. versicolor, S. almum, S. halepense, and Sorghastrum nutans (Yellow Indiangrass) also have the 3.8 kb fragment, and CMS lines studied containing A1, A2 and A3 cytoplasms have the 3.7 kb fragment. The size difference between the two fragments was localized to a 1.0 kb SacI-HindIII fragment by restriction map**. A r65 by deletion, which is flanked by a 51 by tandem repeat, was identified in the CMS lines by sequencing the clones. Comparison of the two sequences with those from maize, rice, tobacco, spinach, pea, and liverwort revealed that the deleted sequence is located in the middle of the RNA polymerase β″ subunit encoded by the gene rpoC2. The amino acid sequence deleted in the CMS lines is in a monocot-specific region which contains two protein motifs that are characteristic of several transcriptional activation factors, namely, a leucine zipper motif and an acidic domain capable of forming an amphipathic α-helix. Further studies designed to determine whether or not the deletion is involved in CMS of sorghum are underway.
Similar content being viewed by others
References
Braun CJ, Siedow IN, Williams ME, Levings CS III (1989) Mutations in the maize mitochondria) T-urf13 gene eliminate sensitivity to a fungal pathotoxin. Proc Natl Acad Sci USA 86:4435–4439
Brown GG, Bussey H, DesRosiers LJ (1986) Analysis of mitochondrial DNA, chloroplast DNA, and double-stranded RNA in fertile and cytoplasmic male-sterile sunflower (Helianthus annuus). Can J Genet Cytol 28:121–129
Bailey-Serres J, Hanson DK, Fox TD, Leaver CJ (1986) Mitochondrial genome rearrangement leads to extension and relocation of the cytochrome c oxidase subunit I gene in sorghum. Cell 47:567–576
Chen K, Kung SD, Gray JC, Wildman SG (1975) Polypeptide composition of fraction 1 protein from Nicotiana glauca and from cultivars of Nicotiana tabacum including a male-sterile line. Biochem Genet 13:771–778
Chen K, Meyer VG (1979) Mutation in chloroplast DNA coding for the large subunit fraction 1 protein correlated with male sterility in cotton. J Hered 70:431–433
Chen K, Sand SA (1979) Nicotiana chromosome coding for a specific polypeptide of the small subunit fraction 1 protein. Science 204:179–180
Chen Z, Liang GH, Muthukrishnan S, Kofoid KD (1990) Chloroplast DNA polymorphism in fertile and male-sterile cytoplasms of sorghum [Sorghum bicolor (L.) Moench]. Theor Appl Genet 80:727–731
Cozens AL, Walker JE (1986) Pea chloroplast DNA encodes homologues of Escherichia coli ribosomal subunit S2 and the β′-sub-unit of RNA polymerase. Biochem J 236:453–460
Dewey RE, Levings CS III, Timothy DH (1986) Novel recombinations in the maize mitochondria) genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell 44:439–449
Dewey RE, Timothy DH, Levings CS III (1987) A mitochondria) protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci USA 84:5374–5378
Duvall MR, Doebley JF (1990) Restriction site variation in the chloroplast genome of Sorghum (Poaceae). Syst Bot 15:472–480
Dwarki VJ, Montminy M, Verma IM (1990) Both the basic region and the “leucine zipper” domain of the cyclic AMP response element binding (CREB) protein are essential for transcriptinal activation. EMBO J 9:225–232
Edwardson RJ (1970) Cytoplasmic male sterility. Bet Rev 36:341–420
Eckenrode VK, Levings CS III (1987) Maize mitochondria) genes and cytoplasmic male sterility. In: Bruening G, Harada J, Kosuge T, Hollaender A (eds) Tailoring genes for crop improvement: an agricultural perspective. Plenum Press, New York, pp 69–84
Flavell R (1974) A model for the mechanism of cytoplasmic malesterility in plants, with special reference to maize. Plant Sci Lett 3:259–263
Frankel R, Scowcroft WR, Whitfeld PR (1979) Chloroplast DNA variation of isonuclear male-sterile lines of Nicotiana. Mol Gen Genet 169:129–135
Galau GA, Wilkins TA (1989) Alloplasmic male sterility in AD alloterraploid Gossypium hirsutum upon replacement of its resident A cytoplasm with that of D species G. harknessii. Theor Appl Genet 78:23–30
Gouyou PH, Vichot F, Van Demme JMM (1991) Nuclear-cytoplasmic male sterility: single-point equilibria versus limit cycle. Amer Nat 137:498–515
Greenberg GM, Narita JO, Deluca-Flaherty C, Gruissem W, Rushlow KA, Hallick RB (1984) Evidence for two RNA polymerase activities in Euglena gracilis chloroplasts. J Biol Chem 259:14880–14887
Gruissem W (1989) Chloroplast gene expression: how plants turn their plastids on. Cell 56:161–170
Hanson MR, Conde MF (1985) Functioning and variation of cytoplasmic genomes: lessons from cytoplasmic-nuclear interaction affecting male fertility in plants. Int Rev Cytol 94:213–267
Hiratsuka J, Shimada H, Whittier R, Ishibashi T, Sakamoto M, Mori M, Kondo C, Honji Y, Sun CR, Meng BY, Li YQ, Kanno A, Nishizawa Y, Hirai A, Shinozaki K, Sugiura M (1989) The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mot Gen Genet 217:185–194
Hu J, Bogorad L (1990) Maize chloroplast RNA polymerase: the 180-, 120-, and 38-kilodalton polypeptides are encoded in chloroplast genes. Proc Natl Acad Sci USA 87:1531–1535
Hudson GS, Holton TA, Whitfeld PR, Bottomley W (1988) Spinach chloroplast rpoBC genes encode three subunits of the chloroplast RNA polymerase. J Mol Biol 200:639–654
Igloi GL, Meinke A, Dory I, Kössel H (1990) Nucleotide sequence of the maize chloroplast rpo B/C1/C2 operon: comparison between the derived protein primary structures from various organisms with respect to functional domains. Mol Gen Genet 221:379–394
Jarl CI, van Grinsven MQJM, van den Mark F (1989) Correction of chlorophyll-defective male-sterile winter oilseed rape (Brassica napus) through organelle exchange: molecular analysis of the cytoplasm of parental lines and corrected progeny. Theor Appl Genet 77:135–141
Johnson PF, McKnight SL (1989) Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem 58:799–839
Kofer W, Glimelius K, Bonnett H (1991) Modifications of mitochondrial DNA cause changes in floral development in homeotic-like mutations of tobacco. Plant Cell 3:759–769
Landschulz WG, Johnson PF, McKnight SL (1988) The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240:1759–1764
Laser KD, Lersten NR (1972) Anatomy and cytology of microsporgenesis in cytoplasmic male sterile angiosperms. Bot Rev 38:425–454
Lerbs S, Brautigam E, Parthier D (1985) Polypeptides of DNA-dependent RNA polymerase of spinach Chloroplasts: characterization by antibody-linked polymerase assay and determination of sites of synthesis. EMBO J 4:1661–1666
Levings CS III, Pring DR (1976) Restriction endonuclease analysis of mitochondrial DNA from normal and Texas cytoplasmic male-sterile maize. Science 193:158–160
Levings CS III (1990) The Texas cytoplasm of maize: cytoplasmic male sterility and disease susceptibility. Science 250:942–947
Little MC, Hallick RB (1988) Chloroplast rpoA, rpoB, and rpoC genes specify three components of a chloroplast DNA-dependent RNA polymerase active in tRNA and mRNA transcription. J Biol Chem 263:14302–14307
Mackenzie SA, Chase CD (1990) Fertility restoration is associated with loss of a portion of the mitochondrial genome in cytoplasmic male-sterile common bean. Plant Cell 2:905–912
Mitchell PJ, Tjian R (1989) Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science 245:371–378
Mullet JE (1988) Chloroplast development and gene expression. Annu Rev Plant Physiol Plant Mot Biol 39:475–502
Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Chang Z, Aota S, Inokuchi H, Ozeki H (1986) Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322:572–574
Palmer JD (1987) Chloroplast DNA evolution and biosystematic uses of chloroplast DNA variation. Amer Nat 130:s6-s29
Palmer JD, Jansen RK, Michaels HJ, Chase MW, Manhart J (1988) Chloroplast DNA variation and plant phylogeny. Ann Missouri Bot Gard 75:1180–1206
Powling A (1982) Restriction endonuclease analysis of mitochondrial DNA from sugarbeet with normal and male-sterile cytoplasms. Heredity 49:117–120
Pring DR, Conde MF, Schertz KF (1982) Organelle genome diversity in sorghum: male-sterile cytoplasms. Crop Sci 22:414–421
Ptashne M (1988) How eukaryotic transcriptional activators work. Nature335:683–689
Ross WM, Hackerott HL (1972) Registration of seven iso-cytoplasmic sorghum germplasm lines. Crop Sci 12:720
Rottmann WH, Brears T, Hodge TP, Lonsdale DM (1987) A mitochondrial gene is lost via homologous recombination during reversion of CMS T maize to fertility. EMBO J 6:1541–1546
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning (2nd ed). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanger F, Coulson AR (1975) A rapid method for determining sequnces in DNA by primed synthesis with DNA polymerase. J Mot Biol 94:441–448
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schertz KF, Pring DR (1982) Cytoplasmic sterility systems in sorghum. In: House LR, Mughogho LK, Peacock JM (eds) Sorghum in the Eighties. Proc Internatl Symp Sorghum, Patancheru, AP India, ICRISAT, pp 373–383
Shimada H, Fukuta M, Ishikawa M, Sugiura M (1990) Rice chloroplast RNA polymerase genes: the absence of an intron in rpoC1 and the presence of an extra sequence in rpoC2. Mot Gen Genet 221:395–402
Shinozaki A, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Meng BY, Sugita M, Deno H, Kamogashira T, Yamada K, Kusuda J, Takaiwa F, Kato A, Tohdoh N, Shimada H, Sugiura M (1986) The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J 5:2043–2049
Sigler PB (1988) Transcriptional activation, acid blobs and negative noodles. Nature 333:210–212
Smith RL, Chowdhury KU (1989) Mitochondrial DNA polymorphism in male-sterile and fertile cytoplasms of pearl millet. Crop Sci 29:809–814
Tewari K, Goel A (1983) Solubilization and partial purification of RNA polymerase from pea chloroplasts. Biochemistry 22:2142–2148
Vedel F, Mathieu C, Lebacq P, Ambard-Bretteville F, Remy R (1982) Comparative macromolecular analysis of the cytoplasms of normal and cytoplasmic male sterile Brassica napus. Theor Appl Genet 62:255–262
Wise RP, Pring DR, Gengenbach BG (1987) Mutation to male fertility and toxin insensitivity in Texas (T)-cytoplasm maize is associated with a frame shift in a mitochondrial open reading frame. Proc Natl Acad Sci USA 84:2858–2862
Zurawski G, Clegg MT, Brown AHD (1984) The nature of nucleotide sequence divergence between barley and maize chloroplast DNA. Genetics 106:735–749
Zurawski G, Clegg MT (1987) Evolution of higher-plant chloroplast DNA-encoded genes: Implications for structure-function and phylogenetic studies. Annu Rev Plant Physiol 38:391–418
Young EG, Hanson MR (1987) A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell 50:41–49
Author information
Authors and Affiliations
Additional information
Communicated by R. Hagemann
Rights and permissions
About this article
Cite this article
Chen, Z., Muthukrishnan, S., Liang, G.H. et al. A chloroplast DNA deletion located in RNA polymerase gene rpoC2 in CMS lines of sorghum. Molec. Gen. Genet. 236, 251–259 (1993). https://doi.org/10.1007/BF00277120
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00277120