Abstract
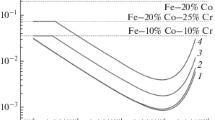
By thermodynamic analysis of oxygen solutions in Fe-Ni-O, Fe-Co-O, and Co-Ni-O melts, the composition of the oxide phase is established for the first time. In addition, the equilibrium oxygen concentrations in these melts are determined over the whole range of alloy compositions. In Fe-Ni-O and Fe-Co-O melts, the oxide phase mainly contains FeO over a relatively broad of alloy compositions. Sharp increase in NiO content is only observed when the molar fraction of nickel exceeds one; sharp increase in CoO content is only observed when the molar fraction of cobalt exceeds 0.8. In the Co-Ni-O system, the oxide phase contains both CoO and NiO over the whole range of alloy compositions. In the Fe-Ni system, adding nickel to the melt reduces the solubility of oxygen as a result of weakening of the oxygen bonds in the melt by nickel and consequent increase in oxygen’s activity. With further increase in nickel content in the melt, the oxygen content rises at first slowly and then very sharply. In the Fe-Co system, analogously, adding cobalt to the melt reduces the solubility of oxygen as a result of weakening of the oxygen bonds in the melt and consequent increase in oxygen’s activity. With further increase in cobalt content, the oxygen content rises at first slowly and then relatively rapidly. In the Co-Ni system, adding nickel to cobalt increases the solubility of oxygen over the whole range of alloy compositions, on account of the significantly greater solubility of oxygen in nickel than in cobalt.
Similar content being viewed by others
References
Steelmaking Data Sourcebook, New York: Gordon & Breach, 1988.
Sigworth, G.K., Elliott, J.F., Vaughn, G., and Geiger, G.H., The thermodynamics of dilute liquid nickel alloys, Metallurgical Society CIM: Annual Volume, 1977, pp. 104–110.
Sigworth, G.K. and Elliott, J.F., The thermodynamics of dilute liquid cobalt alloys, Can. Metall. Quart., 1976, vol. 15, no. 2, pp. 123–127.
Kulikov, I.S., Raskislenie metallov (Deoxidation of Metals), Moscow: Metallurgiya, 1975.
Hultgren, R., Desai, P.D., Hawkins, D.T., et al., Selected Values of the Thermodynamic Properties of Binary Alloys, Metals Park, Ohio: Amer. Soc. Metals, 1973.
Wagner, C., Thermodynamics of Alloys, Cambridge: Addison-Wesley, 1952.
Kubaschewski, O. and Alcock, C.B., Metallurgical Thermochemistry, New York: Pergamon Press, 1967.
Grigoryan, V.A., Belyanchikov, L.N., and Stomakhin, A.Ya., Teoreticheskie osnovy elektrostaleplavil’nykh protsessov (Theoretical Principles of Electric Steelmaking Processes), Moscow: Metallurgiya, 1987.
Averin, V.V., Polyakov, A.Yu., and Samarin, A.M., Solubility and activity of oxygen in liquid iron, nickel, and cobalt and their alloys, Izv. Akad. Nauk SSSR, Otd. Tekhn. Nauk, 1957, no. 8, pp. 120–122.
Dashevskii, V.Ya., Fiziko-khimicheskie osnovy raskisleniya zhelezonikelevykh splavov (Physicochemical Principles of the Reduction of Iron-Nickel Alloys), Moscow: Fizmatlit, 2011.
Frohberg, M.G. and Wang, M., Thermodynamic properties of sulphur in liquid copper-antimony alloys at 1473 K, Z. Metallk., 1990, vol. 81, no. 7, pp. 513–518.
Chiang, T. and Chang, Y.A., The activity coefficient of oxygen in binary liquid metal alloys, Metall. Trans., 1976, vol. 7B, pp. 453–457.
Ishii, F. and Ban-ya, S., Deoxidation equilibrium of silicon in liquid nickel-copper and nickel-cobalt alloys, ISIJ Intern., 1993, vol. 33, no. 2, pp. 245–250.
Dashevskii, V.Ya., Aleksandrov, A.A., and Leont’ev, L.I., Thermodynamics of oxygen solutions in the complex reduction of Fe-Co melts, Steel Transl., 2014, vol. 44, no. 5, pp. 337–344.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.Ya. Dashevskii, A.A. Aleksandrov, L.I. Leont’ev, 2015, published in “Izvestiya VUZ. Chernaya Metallurgiya,” 2015, No. 1, pp. 54–60.
About this article
Cite this article
Dashevskii, V.Y., Aleksandrov, A.A. & Leont’ev, L.I. Thermodynamics of oxygen solutions in Fe-Ni, Fe-Co, and Co-Ni melts. Steel Transl. 45, 42–48 (2015). https://doi.org/10.3103/S0967091215010052
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0967091215010052