Abstract
Selenium nanocrystals (SeNPs) were developed using Coccinia grandis fruit (CGF) extract by green microwave approach. The morphological characters revealed that the quasi SeNPs with dimensions ranging from 12 to 24 nm have been arranged in encapsulated spherical geometries with dimensions ranging from 0.47 to 0.71 m. The DPPH assay revealed that SeNPs at a concentration of 70 µl of 99.2% had the greatest possible scavenging capacity. The nanoparticle concentrations were around 500 g/mL and the cellular uptake of SeNPs through living thing extracellular matrix cell lines in vitro was limited to 75.1 ± 3.8%. The biocidal activity was tested against E. coli, B. cereus, and S. aureus strains. Since it had the greatest MIC against B. cereus with 32 mm compared with the reference antibiotics. These incredible qualities of SeNPs suggest that attempting to manipulate multi-purpose nanoparticles for powerful and flexible wound and skin therapeutic innovations is very impressive.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotic resistance will become one of the maximum critical complications that acute wounds can cause [8]. Moreover, it is approved by the US Food and Drug Administration for use as a routine food additive [9, 10] and is recommended for cardiovascular disease prevention. It is an essential nutrient of multiple antioxidant enzymes, including glutathione peroxidase and thioredoxin reductase, that also alleviate the free radicals that cause [11]. In the internal organs, se is accumulated with at least 25 enzyme systems that perform a variety of functions such as anti-inflammatory, antioxidant, antibiotic, and anti-carcinogenic agents [12]. Furthermore, this component may be engaged in the oxidative stress of nucleophiles in protein complexes, including glycine dehydrogenase and protein kinase C [13]. It's indeed possible that a lack of Se may decrease bone density as well as collagen formation. In current history, there has been an increase in the number of attractive studies focusing on the synthesis of selenium nanoparticles (Se NPs), while these nanoparticles possess impressive biological functions (in vitro and in vivo), low toxicity, and outstanding biocompatibility [14] As a matter of fact, these SeNPs may take part in antioxidant defense mechanisms and play a major role in oxidative stress protection [15, 16] A few studies have identified that Selenium NPs are much more beneficial and far less toxic than silver nanoparticles (Ag-NPs). Furthermore, the chemical stability of Selenium NPs is thought to be greater than those of Ag-NPs. It had been proposed that Selenium NPs could impede coronavirus insertion across health cells, thereby eliminating their infective behavior [17]. The distinctive antibacterial effect of Selenium NPs is highly reliant on the preparation method and may be governed by cell-cycle homeostases such as reactive oxidative species (ROS) expulsion and various enzyme signal processing. Selenium NPs have tremendous potential in anticancer therapy because they impede cancer cell proliferation and also have cellular membranes toxic effects. Green chemistry approaches were used to create globular Selenium nanoparticles from bioactive components like vitamin c or methanolic extracts, and they demonstrated superior antibacterial agents when compared to those created utilizing chemical treatment [18, 19]. Furthermore, the physicochemical characteristics of Selenium NPs may be influenced by the economic circumstances of the preferred biosynthetic pathway. The main drawbacks of physicochemical methods include not only high time and financial usage, and yet also the use of numerous harmful chemicals that may be applied to the surface of SeNPs, thereby limiting their pharmaceutical and biomedical applications [20]. Plant-mediated and eco-friendly attitudes, which are a critical aspect in the fabrication of SeNPs, overcame these challenges. For instance, nano-sized Selenium NPs were created by combining Withania somnifera aqueous leaf extract with 50 mM selenious acid [21]. The formulation of Selenium NPs using Coccinia Grandi’s flower as a bioactive source is acceptable and has almost zero toxic types, unlike nanoparticles acquired through chemical agents. Besides that, the water-soluble polymer component can be used as a strong stabilizer in the fabrication of Selenium colloidal particles [22]. The paper design features and identifies environmentally friendly and simple-to-use approaches for the development of Selenium nanoparticles employing Coccinia Grandi’s flower extract as a stabilizer. The structural performance, microstructural, electro-optic, and morphological characteristics of greenly synthesized Selenium nanoparticles could be investigated. In addition, to using a human extracellular cell line, the cell debris in the presence of Selenium NPs was evaluated (HFB-4). To verify the antimicrobial effects of Selenium NPs, gram-positive and gram-negative bacterial strains S. Aureus and E. Coli were preferred as acute gram-positive and gram-negative bacterial strains, respectively, and antioxidant capability was measured by using the DPPH assay.
Results and discussion
Characteristics of the electromagnetic spectrum
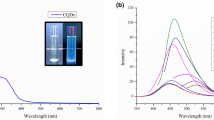
The transformation of orange to red solid, which has been associated to surface Optical Absorption (SPR), impressive photonic properties for metallic NPs, and the lighting adjustment indicates that Selenium NPs are developed [Fig. 1]. Ultra-violet visible spectroscopy (UV–Vis) has been used to examine the bio-induced Selenium NPs, the intensity of which was approximated to be between 200 and 800 nm. The highest absorption spectrum at 261 nm is the result of conscious vibrations of free electrons that are accelerated and constrained on one exterior of the Se moiety to another, as determined by their Surface plasmon resonance. Furthermore, the coloring remained steady for 1 day after a total response, when no patterns were observed. Selenium NPs, in distinctive, have characteristics that seem to be dependent on size and morphology. Because of the concept of constructed conventions and the quantum constraint influence, Selenium NPs also demonstrated a significant number of integration communities nearer to the UV–Vis zone. Hassanien et al. [23] used Drumstick watery ingredients to develop Selenium NPs, and the Surface plasmon resonance band has been outstanding at 390 nm. Nevertheless, a study conducted by Kirupagaran et al. revealed that intake at 293 nm was a characteristic of SeNPs [24]. In photons of this subsector, the prevailing absorption evidence is much more closely linked to the previous prose.
FTIR spectral investigation
The FTIR spectroscopic layout has been carried out to depict the useful gatherings observed in the colloidal form of SeNPs encapsulated in the CGF extract [Fig. 2]. Due to various CGF/Selenium NPs, the assimilation group at 3442 cm−1 denotes the CGF's expanded recurrence of –OH groups and is additionally allotted as–OH on the Selenium NPs exterior. The band seen around 1641 cm−1 corresponds to C=O stretched vibration, whereas the one seen at 1384.1 cm−1 corresponds to the second NH2 bunch and symmetric vibrations of CH, respectively [25]. The FTIR confirms the drastic reduction in Se. The factions at 821 and 523 cm−1 correspond to the reduction of Selenium NPs with protonated groups as Se–O as a sign of cohesion interconnection between Se and ascorbic corrosive. It should be noted that the reaction increases with decreasing molecule size, so the materials should be surrounded to avoid receptor uptake. Furthermore, various coverage professionals have been used to resolve Selenium NPs. Selenium molecules were established by CGF in this evaluation, as shown by the FTIR findings.
Crystal structure and thermal stability identification
A powder X-ray pattern [Fig. 3(a)] is an important method for detecting the crystalline substance phase. The crystallographic signal and their essence planes were documented at locations 2θ = 22.88° (100), 29.28° (101), 40.9° (110), 43.36° (102), 51.12° (112), 55.38° (202), as well as 60.88° (210), suggesting the occurrence of Selenium nanoparticles in a perfect crystalline configuration as well as approving with Joint committee on powder diffraction No. 06-0362. The incredible peak located at 2θ = 29.28° (101), evidenced that a considerable angle occurred to the evaluated component (101) and also illustrated the greater arrangement of Selenium NPs upon structure. The Debye–Scherer condition (1) could be used to evaluate the average crystalline dimensions [26].
As K is the Scherer consistent (0.9), λ is the frequency of the X-beam, β is the full-width half maximum of the XRD peak and θ is the Bragg angle. Accordingly, the determined crystallite size is around 27 nm. It very well may be expressed as a hexagonal structure.
TGA analysis of the green-arranged Selenium nanoparticles was performed and is shown in [Fig. 3(b)]. The curve pattern reveals a weight loss of 1.3% up to 250 °C, possibly as a result of debased breakthroughs substance (mostly adsorbed with moisture), and its weight reduction is by all accounts nearly perfect after about 465 °C. It could be referred to as the N2 streaming condition that caused the vaporization of Selenium nanoparticles.
Microstructural and morphological characteristics
The surface morphological spectrum of Selenium nanoparticles is shown in [Fig. 4(a)]. These SEM micrographs revealed granular shapes of polydispersed selenium nanoparticles. The average area is calculated to be between 15 and 30 nm. Figure 4(b and c) depict the morphology of selenium nanoparticles (c). The TEM images of Se@CGF NPs show spherical patterns with particles ranging in size from 12 to 22 nm. Besides that, as seen in [Fig. 4(d)], the polycrystalline structures of green synthesized Selenium nanoparticles were evidenced by their correlating SAED assessment, with readily identifiable peaks (100), (101), (110), (102), (112), (202), and (210), crystalline shapes suggesting hexagonal structure strategy. The HR-TEM image also shows a well-defined inner surface of the ligament, with the d –widths for adjacent area adhesive fringes estimated to be 0.256 nm, which corresponds to the proportion of the (101) plane of selenium nanoparticles.
Furthermore, the EDX study displayed the essential Se. The EDX investigation provides a quantitative and subjective assessment of the constituents that may be engaged in the creation of nanostructures. Figure 5 depicts a profile of a nanoparticle component made with green ascorbic corrosive. With a percentage contribution of 53.4%, the crucial Se demonstrated an electronic serious retention peak at around 1.45 keV.
In vitro antiradical activity
Free radical scavenging activity is a strong oxidant that is well for its ability to reduce the acceptability of hydrogen or electron from donations. The discoloration of the CGF@SeNPs was used to evaluate their DPPH-reducing behavior. When particularly in comparison to the leaf extract the free radical scavenging activity inhibited CGF@SeNPs operation significantly [Fig. 6(a)]. The DPPH action of selenium nanoparticles was strong and chosen depending. As a result, the CGF@SeNPs demonstrated greater inhibitory effects, to 99% DPPH scavenging activity. After incorporating CGF@SeNPs into the DPPH solution, the color intensity was attributed to the effect of DPPH scavenging caused by the donor of a hydrogen ion to stabilize the DPPH molecule [27, 28]. CGF@SeNPs antioxidant capacity might be credited to the operational group to them, which was derived from the leaf extracts.
In vitro cell viability of synthesized selenium nanoparticles
The cytotoxic effect of Selenium nanoparticles was assessed in vitro on a living creature fibroblast cell line (HFB-4). This was accomplished to understand the potential cytostatic mobility of varied Selenium nanoparticle drugs. As seen in [Fig. 6(b)], increasing the drug of Selenium nanoparticles reduced cell plausibility significantly. It started at 97.3 ± 3.1% for the uncontrolled cell line (control one) and fell to 75.1 ± 3.8% for the highest concentration of Selenium nanoparticles, which was around 500 g/mL. Selenium nanoparticles have such a strong effect on cell lines rapidly with the advent of nano-colloidal particles through mammalian cells, which may operate with Oxidative to interact with cell films. This circumstance may crumble existing cells, due to a greater rate of cell death. Also, the size, distribution, and crystalline structure of SeNPs all play a role in their cytotoxic effects [29]. This seems to be because reduced nanoparticles have such a significant increase of corruption, which leads to an increase in the effective drug of those nanoparticles. As a result, influencing poisonous behavior ought to be massively feasible through agreement ailments.
Effect of antibacterial activity
The antibacterial effects of the synthesized Selenium nanoparticles and fruit extract of Coccinia grandis (CGF) and against three separate bacterial pathogens were investigated, which include Gram-positive Staphylococcus aureus (ATCC 43,300), Bacillus cereus (ATCC 11,778), and Gram-negative bacteria Escherichia coli (ATCC 35,218). As displayed in [Fig. 7(a–d)], SeNPs constructed from CGF extract possess a stronger antibacterial effect but extracts have inadequate activity against all the tested bacterial species. These findings support those of Acay et al. [30] and Logaranjan et al. [31]. Critical action was seen at three fixations (50 μL, 75μL, and 100 μL) of the combined SeNPs utilizing the CGF separate against E. coli, B. cereus, and S. aureus with zones of restraint of 10, 16, and 22 mm, 12, 22, and 30 mm, and 35, 40, and 48 mm, individually [Fig. 7(a and b)]. The CGF removal shows action against E. Coli and S. Aureus with zones of the hindrance of 10 and 12 mm and for B. Cereus there was no zone of hindrance, as displayed in Fig. 7. Minimum inhibitory concentrations (MICs) of synthesized Selenium nanoparticles against bacterial pathogens (Table 1) were calculated by using the following formula,
Mechanisms of bactericidal effect
Selenium nanoparticles (Se NPs) from removes display wonderful antibacterial behavior and could be explained by the way that exceptionally unequivocal phytoactive mixtures covered the Se NPs from the plant separately. These phytochemicals that may proceed as lessening as well as covering specialists would be well engaged with the decrease of Selenium nanoparticles into selenium metal and the phytochemical-helped blend of Selenium nanoparticles from the plant separate for nucleation, adjustment, and covering [32]. Thusly, the combined Selenium nanoparticles further developed the antimicrobial movement inferable from the molecule size and the collection of NPs [30] Also, past examinations show that the antibacterial movement relies upon the portion of the combined Se NPs. Also, past examinations show that the antibacterial movement relies upon the portion of the combined Selenium nanoparticles. From Fig. 7, it very well may be noticed that the high convergence of Selenium NPs causes more antibacterial action and bacterial cell demise, because of the association among nanoparticles and the protein of microscopic organisms. Generally, the connection of fabricated Selenium NPs and microbial species can be disclosed because of the interface between positive charges on the microorganism’s surface and negative charges in NPs and the ejection of intracellular material that actuates cell passing because of physicochemical alterations in the bacterial cell divider, bringing about the NPs halting DNA replication and breath of microbes [33, 34]
Conclusion
A sustainable and environmental approach was used to construct selenium nanocrystals (SeNPs). The nanoparticles were determined and assessed for antioxidant and antibacterial behavior. The crystallite size of the as-synthesized SeNPs was about 37 nm and TEM analysis revealed that the Selenium NPs were monodisperse and quasi in shape, with sizes ranging from 12 to 24 nm. SEM morphological analysis revealed that Se NPs have been shaped in crystallized spherical shapes. The anti-oxidant test was performed using DPPH free radical scavenging, which revealed that SeNPs with an accumulation of 99.2% had the greatest possible scavenging ability (99.2 and 200 µl) Besides that, the biomedical appraisal of Se NPs derived from green synthesis revealed that they have excellent bactericidal attributes against E. coli, B. cereus, and S. aureus, all of which are pathogenic organisms frequently encountered in contagious skin disorders. As an outcome of the diversity of green synthesized Se NPs as bioactive molecules, they are a desirable associate for attempting to control infectious disease and beneficial in other pharmacological sectors.
Experimental
Materials and reagents collection
We accumulated healthy Coccinia grandis fruits (CGF) collected from the Sarabanga River in Omalur, Salem District, Tamil Nadu, and India. Merck provided sodium selenite (Na2SeO3) and ethanol. All of the testing methods were executed by washing with sterile.
Preparation of natural reducing agent (NRA)
The Coccinia grandis fruit (CGF) has been washed thoroughly in water to eradicate dirt and debris before being shade oxidized. The Coccinia grandis fruit (CGF) extract was made by combining 1 g of powdered fine with 500 ml of de-ionized water in a 500-ml beaker. For 1 h, the mixture was subjected until the colorless aqueous solution turned yellow. The combination was left at room temperature before getting refracted with (Whatman no. 1) filter paper and centrifuged at 1500 rpm for 5 min to eliminate biomaterials. The isolate was kept at 25 °C to be utilized for the subsequent analyses.
Green-influence synthesis of Se NPs
The design and construction of SeNPs were attained using Na2SeO3 and Coccinia grandis fruit (CGF) extract as a cap** and stabilizing agent, as shown in Fig. 8. Sodium selenite solutions (30 mM) and stabilizing agents (as-prepared 1 g of CGF at 90 °C for 4 h) were mixed at room temperature (R.T) for 15 min with continuous agitation to generate the aqueous solution of stabilized SeNPs. After that, the mixture was poured into a Teflon-lined STRT SYNTH microwave reactor (600 W, 70% stirring, 80 °C, and 1Par). The mixture's color was observed from colorless to orange, which was credited to the development of SeNPs. Finally, the homogenate was stirred at 8000 rpm to separate the Se NPs. The Nanoparticles were sterilized by rinsing them with DDI several times and then with 100% alcohol, then exfoliating them in a 50 °C microwave for 24 h to obtain homogeneous darkened particles [35].
Anti-radical activity
DPPH (2,2-diphenyl-2-picrylhydrazyl hydrate) study was used to determine the free radical scavenging capacity of SeNPs [4]. Specifically, different doses of SeNPs (0, 50, 100, 150, and 200 µl/mL) were regarded as separate with 2 mL of a 0.2 solution mM of DPPH in methanol solvent, blended thoroughly, and afterward incubated for 30 min in the shade. The materials' absorbance was measured at 517 nm with the previously stated twin-beam UV–vis. The antioxidant activity was assessed using the formula (3)
Evaluation of cell viability in the presence of Selenium nano-colloids
The cell growth in the existence of Selenium NPs was predicted using the MTT assessment [36], which also represents the difference between yellow dye (3- (4,5-dimethylthiazol-2-yl) 2.5- diphenyltetrazolium bromide) to translucent formazan crystal influence the activity of cell membrane cyclooxygenase and succinyl oxidative metabolism enzymatic in living organisms [37]. Upon fertilization in Dulbecco's modified Medium containing (DMEM, Gibpco) at 37 °C in a 5% CO2 incubator, the cells were seeded in 96-well plates and incubated of 1 104 cells/cm2 and seeded in 96-well plates. The mixture was incubated for 1 day at 37 °C in a 5% CO2 incubator. After separating the old media, 200 m of various concentrations of Selenium NPs dissolved in the medium were added to HFB-4 cells. In the medicated wells, the ultimate accumulation was 0.0, 100, 200, 300, 400, and 500 ppm. Cell convergence was determined after 1 day of exposure. After adding a 20 L remedy of MTT in PBS (5 mg/mL) to every well, the plates were treated for another 4 h at 37 °C in a humid environment with 5% CO2. The permeate was collected and excluded, the Formosan crystals were disintegrated in 100 L of DMSO, and the optical intensity at 570 nm was determined. The experimental studies were substantiated three times, and the cytotoxic effect was calculated using the Eqs. (4) [38, 39].
Bactericidal activity
This evaluation used three distinct types of microbial species, such as Escherichia coli ATCC35218, Staphylococcus aureus ATCC 43,300, and Bacillus cereus ATCC 11,778 (clinical disengage), all obtained from ST. Joseph College, Trichy. The antibacterial effect of the conceived Se NPs was investigated in the present study using a very delicate displacement tactic. 14 g of nutrition agar were segmented in 500 mL of deionized water and pasteurized for 45 min at 121 °C and 15 psi. To develop a well, a germ-free plug course was used to puncture the potent mode (4 wells in each plate). The extract of fruits was utilized as a control, and three various dosages of Se NPs (50 μL, 75μL, and 100 μL) have been used, accompanied by an increasing accumulation till the gap was loaded and fertilized at 37 °C for 24 h. Upon fermentation, the domain was measured and described in mm of the radius of curvature. [40, 41].
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
03 April 2023
A Correction to this paper has been published: https://doi.org/10.1557/s43578-023-01001-0
References
Y. Zou, R. **e, E. Hu, P. Qian, B. Lu, G. Lan et al., Protein-reduced gold nanoparticles mixed with gentamicin sulfate and loaded into konjac/gelatin sponge heal wounds and kill drug-resistant bacteria. Int. J. Biol. Macromol. 148, 921–931 (2020)
A.A. Menazea, M.K. Ahmed, Wound healing activity of Chitosan/Polyvinyl Alcohol embedded by gold nanoparticles prepared by nanosecond laser ablation. J. Mol. Struct. 1217, 128401 (2020)
A.A. Menazea, M.K. Ahmed, Nanosecond laser ablation assisted the enhancement of antibacterial activity of copper oxide nano particles embedded though Polyethylene Oxide/ Polyvinyl pyrrolidone blend matrix. Radiat. Phys. Chem. 174, 108911 (2020)
A.A. Menazea, M.K. Ahmed, Silver and copper oxide nanoparticles-decorated graphene oxide via pulsed laser ablation technique: preparation, characterization, and photoactivated antibacterial activity. Nano Struct. Nano Object 22, 100464 (2020)
A.A. Menazea, M.K. Ahmed, Synthesis and antibacterial activity of graphene oxide decorated by silver and copper oxide nanoparticles. J. Mol. Struct. 1218, 128536 (2020)
V. Jeevanantham, D. Tamilselvi, S.R. Bavaji, S. Mohan, Green formulation of gold nanoparticles and their antioxidative assays, antimicrobial activity and photocatalytic colour decay. Bull. Mater. Sci. 46, 32 (2023)
Z. Qiu, Q. Tian, T. Zhang, D. Yang, F. Qiu, Fabrication of dynamic zero-valent iron/MnO2 nanowire membrane for efficient and recyclable selenium separation. Separ. Purif. Technol. 230, 115847 (2020)
S. Satheeskumar, V. Jeevanantham, D. Tamilselvi, Effect of Cu-do** on the structural and optical properties of ZnO nanocrystallites prepared by chemical precipitation method. J. Ovon. Res 14, 9–154 (2018)
V. Jeevanantham, K. Hemalatha, S. Satheeskumar, Photodegradation activity of pure PVP capped and chitosan capped ZnO nanoparticles against azo red dye under UV irradiation. J. Ovonic Res. 14, 269–275 (2018)
Y. Wang, W. He, H. Hao, J. Wu, N. Qin, Eggshell derived Sedoped HA nanorods for enhanced antitumor effect and curcumin delivery. J. Sol. Gel. Sci. Technol. 87(3), 600–607 (2018)
A. Caglayan, D.C. Katlan, Z.S. Tuncer, K. Yuce, Evaluation of trace elements associated with antioxidant enzymes in blood of primary epithelial ovarian cancer patients. J Trace Elem. Med. Biol. 52, 254–262 (2019)
R. Alhasan, A. Kharma, P. Leroy, C. Jacob, C. Gaucher, Selenium donors at the junction of inflammatory diseases. Curr. Pharmaceut. Des. 25(15), 1707–1716 (2019)
M. Farina, M. Aschner, Glutathione antioxidant system and methylmercury-induced neurotoxicity: an intriguing interplay. Biochim Biophys Acta Gen. Subj. 1863(12), 129285 (2019)
P. Adadi, N.V. Barakova, K.Y. Muravyov, E.F. Krivoshapkina, Designing selenium functional foods and beverages: a review. Food Res. Int. 120, 708–725 (2019)
S. Rao, Y. Lin, Y. Du, L. He, G. Huang, B. Chen et al., Designing multifunctionalized selenium nanoparticles to reverse oxidative stress-induced spinal cord injury by attenuating ROS overproduction and mitochondria dysfunction. J. Mater. Chem. B 7(16), 2648–256 (2019)
K.A. Amin, K.S. Hashem, F.S. Alshehri, S.T. Awad, M.S. Hassan, Antioxidant and hepatoprotective efficiency of selenium nanoparticles against acetaminophen-induced hepatic damage. Biol. Trace Elem. Res. 175(1), 136–145 (2017)
M. Kieliszek, B. Lipinski, Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 143, 109878 (2020)
G. Zhang, L. Zhou, D. Cai, Z. Wu, Anion-responsive carbon nanosystem for controlling selenium fertilizer release and improving selenium utilization efficiency in vegetables. Carbon 129, 711–719 (2018)
X. Wang, D. Zhang, X. Pan, D.J. Lee, F.A. Al-Misned, M.G. Mortuza et al., Aerobic and anaerobic biosynthesis of nano-selenium for remediation of mercury contaminated soil. Chemosphere 170, 266–273 (2017)
P.G. Jamkhande, N.W. Ghule, A.H. Bamer, M.G. Kalaskar, Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 53, 101174 (2019)
V. Alagesan, S. Venugopal, Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanosci 9(1), 105–116 (2019)
P.A. Tran, N. O’Brien-Simpson, J.A. Palmer, N. Bock, E.C. Reynolds, T.J. Webster et al., Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillinresistant Staphylococcus aureus and epidermidis: in vitro and in vivo assessment. Int. J. Nanomed. 14, 4613 (2019)
R. Hassanien, A.A. Abed-Elmageed, D.Z. Husein, Eco-friendly approach to synthesize selenium nanoparticles: photocatalytic degradation of sunset yellow azo dye and anticancer activity. Chemistry 4(31), 9018–9026 (2019)
R. Kirupagaran, A. Saritha, S. Bhuvaneswari, J. Nanosci. Technol. 2(5), 224–226 (2016)
W. Yanhua, H. Hao, Y. Li, S. Zhang, Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloids Surf. B Biointerfaces 140, 297–306 (2016)
K. Shoueir, M.K. Ahmed, S.A. Abdel Gaber, M. El-Kemary, Thallium and selenite doped carbonated hydroxyapatite: microstructural features and anticancer activity assessment against human lung carcinoma. Ceram. Int. 46(4), 5201–5212 (2020)
P. Molyneux, The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 26(2), 211–219 (2004)
N. Kanipandian, S. Kannan, R. Ramesh, P. Subramanian, R. Thirumurugan, Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater. Res. Bull. 49, 494–502 (2014)
V.L.C. Tan, A. Hinchman, R. Williams, P.A. Tran, K. Fox, Nanostructured biomedical selenium at the biological interface (Review). Biointerphases 13(6), 06D301 (2018)
H. Acay, Biosynthesis and characterization of silver nanoparticles using fig (ficus carica) leaves: a potential antimicrobial activity. Appl. Ecol. Env. Res. 17, 13793–13802 (2019)
K. Logaranjan, S. Devi, K. Pandian, Biogenic synthesis of silver nanoparticles using fruit extract of ficus carica and study its antimicrobial activity. Nano Biomed. Eng. 4, 177–182 (2012)
M.A. Awad, M.M. Alkhulaifi, N.S. Aldosari, S. Alzahly, A. Aldalbahi, Novel eco-synthesis of PD silver nanoparticles: characterization, assessment of its antimicrobial and cytotoxicity properties. Materials 12, 3890 (2019)
K. Espoir, A. Kambale, I. Christian, A.B. Nkanga, I. Blaise-Pascal, A. Mutonkole et al., Memvanga green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 6, e04493 (2020)
Q.L. Feng, J. Wu, G.Q. Chen, F.Z. Cui, T.N. Kim, J.O. Kim, A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and taphylococcus aureus. J. Biomed. Mater. Res. 52, 662–668 (2000)
S. Boroumand, M. Safari, E. Shaabani, M. Shirzad, Majidi R. Faridi, Selenium nanoparticles: synthesis, characterization and study of their cytotoxicity, antioxidant and antibacterial activity. Mater. Res. Express 6(8), 0850d8 (2019)
V. Ruiz-Torres, C. Rodrı´guezperez, M. Herranz-Lopez, B. MartınGarcı´a, A.M. Gomez-Caravaca, D. Arraez-Roman et al., Marine invertebrate extracts induce colon cancer cell death via ROS-mediated DNA oxidative damage and mitochondrial impairment. Biomolecules 9(12), 771 (2019)
S.F. Mansour, R. Al-Wafi, M.K. Ahmed, S. Wageh, Microstructural, morphological behavior and removal of Cr(VI) and Se(IV) from aqueous solutions by magnetite nanoparticles/PVA and cellulose acetate nanofibers. Appl Phys A (2020). https://doi.org/10.1007/s00339-020-3377-z
R. Al-Wafi, S.F. Mansour, M.K. Ahmed, Mechanical, microstructural properties and cell adhesion of Sr/Sehydroxyapatite/graphene/polycaprolactone nanofibers. J. Thermoplast. Compos. Mater. (2020). https://doi.org/10.1177/089270572091278
M.K. Ahmed, A.A. Menazea, A.M. Abdelghany, Blend biopolymeric nanofibrous scaffolds of cellulose acetate/ epsilon-polycaprolactone containing metallic nanoparticles prepared by laser ablation for wound disinfection applications. Int. J. Biol. Macromol. 155, 636–644 (2020)
N. AlMasoud, H. Alhaik, M. Almutairi, A. Houjak, K. Hazazi, F. Alhayek, S. Aljanoubi, A. Alkhaibari, A. Alghamdi, D.A. Soliman, T.S. Alomar, M.A. Awad, Green nanotechnology synthesized silver nanoparticles: Characterization and testing its antibacterial activity. Green ProcesS. Synth (2021). https://doi.org/10.1515/gps-2021-0048
V. Jeevanantham, K.V. Hemalatha, S. Satheeskumar, Enhanced antimicrobial activities of hybrid ZnMgAlO nanocomposite by soft chemical method. J. Adv. Chem. 12, 5730–5735 (2017)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeevanantham, V., Tamilselvi, D., Rathidevi, K. et al. Greener microwave synthesized Se nanospheres for antioxidant, cell viability, and antibacterial effect. Journal of Materials Research 38, 1909–1918 (2023). https://doi.org/10.1557/s43578-023-00965-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-00965-3