Abstract
Background
Resection remains the cornerstone of curative-intent treatment for biliary tract cancers (BTCs). However, recent randomized data also support a role for adjuvant chemotherapy (AC). This study aimed to characterize trends in the use of AC and subsequent outcomes in gallbladder cancer and cholangiocarcinoma (CCA).
Methods
The National Cancer Database (NCDB) was queried for patients with resected, localized BTC from 2010 to 2018. Trends in AC were compared among BTC subtypes and stages of disease. Multivariable logistic regression was used to identify factors associated with receipt of AC. Survival analysis was performed with Kaplan-Meier and multivariable Cox proportional hazards methods.
Results
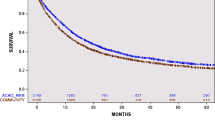
The study identified 7039 patients: 4657 (66%) with gallbladder cancer, 1159 (17%) with intrahepatic CCA (iCCA), and 1223 (17%) with extrahepatic CCA (eCCA). Adjuvant chemotherapy was administered to 2172 (31%) patients, increasing from 23% in 2010 to 41% in 2018. Factors associated with AC included female sex, year of diagnosis, private insurance, care at an academic center, higher education, eCCA (vs iCCA), positive margins, and stage II or III disease (vs stage I). Alternatively, increasing age, higher comorbidity score, gallbladder cancer (vs iCCA), and farther travel distance for treatment were associated with reduced odds of AC. Overall, AC was not associated with a survival advantage. However, subgroup analysis showed that AC was associated with a significant reduction in mortality among patients with eCCA.
Conclusions
Among the patients with resected BTC, those who received AC were in the minority. In the context of recent randomized data and evolving recommendations, emphasis on guideline concordance with a focus on at-risk populations may improve outcomes.



Similar content being viewed by others
References
DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. https://doi.org/10.1097/01.sla.0000251366.62632.d3.
Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol. 2015;33:1845–8. https://doi.org/10.1200/jco.2014.59.7591.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. https://doi.org/10.3322/caac.21590.
Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934–40. https://doi.org/10.1200/jco.2011.40.5381.
Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105:192–202. https://doi.org/10.1002/bjs.10776.
Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol. 2019;37:658–67. https://doi.org/10.1200/jco.18.00050.
Bridgewater J, Fletcher P, Palmer DH, et al. Long-term outcomes and exploratory analyses of the randomized phase III BILCAP study. J Clin Oncol. 2022;40:2048–57. https://doi.org/10.1200/jco.21.02568.
Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. J Clin Oncol. 2017;35(15 Suppl):4006–4006. https://doi.org/10.1200/JCO.2017.35.15_suppl.4006.
Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–73. https://doi.org/10.1016/s1470-2045(18)30915-x.
Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37:1015–27. https://doi.org/10.1200/jco.18.02178.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. Published 2022. Updated 15 July 2022. Retrieved 10 September 2022 at https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
Yoo C, Shin SH, Park JO, et al. Current status and future perspectives of perioperative therapy for resectable biliary tract cancer: a multidisciplinary review. Cancers. 2021;13:1647. https://doi.org/10.3390/cancers13071647.
Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722–8. https://doi.org/10.1001/jamaoncol.2016.6905.
Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488–90. https://doi.org/10.1002/jso.21173.
Mitin T, Enestvedt CK, Jemal A, et al. Limited use of adjuvant therapy in patients with resected gallbladder cancer despite a strong association with survival. JNCI J Natl Cancer Inst. 2017;109:djw324. https://doi.org/10.1093/jnci/djw324.
Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–20. https://doi.org/10.1258/jrsm.2011.110180.
Sohal DP, Shrotriya S, Abazeed M, et al. Molecular characteristics of biliary tract cancer. Crit Rev Oncol Hematol. 2016;107:111–8. https://doi.org/10.1016/j.critrevonc.2016.08.013.
Jusakul A, Cutcutache I, Yong CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7:1116–35. https://doi.org/10.1158/2159-8290.Cd-17-0368.
Brandi G, Farioli A, Astolfi A, et al. Genetic heterogeneity in cholangiocarcinoma: a major challenge for targeted therapies. Oncotarget. 2015;6:14744–53. https://doi.org/10.18632/oncotarget.4539.
Pellino A, Loupakis F, Cadamuro M, et al. Precision medicine in cholangiocarcinoma. Transl Gastroenterol Hepatol. 2018;3:40. https://doi.org/10.21037/tgh.2018.07.02.
Chen Y, Zhang B, Liu C, et al. Clinical efficacy of adjuvant treatments for patients with resected biliary tract cancer: a systematic review and network meta-analysis. BMJ Open. 2022;12:e051421. https://doi.org/10.1136/bmjopen-2021-051421.
Acknowledgment
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC’s NCDB are the source of the de-identified data used in this study. They have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
K. Rhodin is supported by NIH 1R38AI140297. A. Liu is supported by NIH F32CA268527. K. Rhodin and A. Liu both acknowledge support from the Duke Cancer Institute as part of the P30 Cancer Center Support Grant (grant ID: P30 CA014236). M. Lidsky receives funding support from the Department of Surgery and Duke Cancer Institute, the Duke School of Medicine Strong Start Award, The American College of Surgeons National Surgeon Scientist Program, and the Cholangiocarcinoma Foundation Research Fellowship Award. This project also was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant KL2 TR002554 (M. Lidsky). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. J. Strickler declares Abbvie: consulting (self); research grant (institution). Amgen: consulting (self); research grant (institution). AstraZeneca: consulting (self); research grant (institution). Beigene: consulting (self); research grant (institution). Daiichi-Sankyo: consulting (self); research grant (institution). Eli Lilly: consulting (self); research grant (institution). GSK: consulting (self); research grant (institution). Natera: consulting (self). Pfizer: consulting (self). Roche/Genentech: consulting (self); research grant (institution). Seagen: consulting (self); research grant (institution). Takeda: consulting (self). Viatris: consulting (self). Silverback Therapeutics: consulting (self); research grant (institution). Astar D3: research grant (institution). Bayer: consulting (self); research grant (institution). Curegenix: research grant (institution). Gossamer Bio: research grant (institution). Leap Therapeutics: research grant (institution). Nektar: research grant (institution). Sanofi: research grant (institution).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Oral Presentation at the Americas Hepato-Pancreato-Biliary Association Annual Meeting, March 10, 2023, Miami, FL.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rhodin, K.E., Liu, A., Bartholomew, A. et al. Trends in Receipt of Adjuvant Chemotherapy and its Impact on Survival in Resected Biliary Tract Cancers. Ann Surg Oncol 30, 4813–4821 (2023). https://doi.org/10.1245/s10434-023-13567-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13567-4