Abstract
Background
The degree of metabolic activity in tumor cells can be determined by 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET). Associations between FDG uptake by primary tumors of locally advanced esophageal squamous cell carcinoma (ESCC) under trimodal therapy and the pathological features of such tumors have not been fully investigated.
Patients and Methods
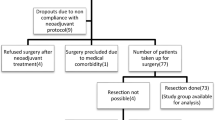
We evaluated relationships between the maximal standardized uptake (SUVmax) in primary tumors on preoperative PET images and pathological features as well as cancer recurrence in 143 patients with ESCC who underwent neoadjuvant chemoradiotherapy (NCRT) followed by surgery.
Results
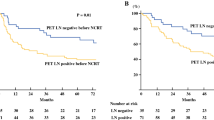
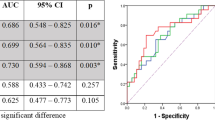
The post-SUVmax significantly differed after NCRT for ypT and ypN status, lymphatic invasion (LI), venous invasion (VI), and recurrence. Furthermore, the %ΔSUVmax (rate of decrease between before and after NCRT) for LI, VI, and recurrence significantly differed. Univariate and multivariate analyses selected post-SUVmax and %ΔSUVmax as independent preoperative predictors of recurrence-free survival [hazard ratio (HR) 1.46; 95% confidence interval (CI) 1.24–1.72 and HR 0.97; 95% CI 0.96–0.99, respectively; p < 0.001 for both]. Recurrence-free and overall survival were significantly stratified according to optimal SUVmax cutoffs for predicting recurrence (post- and %ΔSUVmax: 2.8 and 70, respectively).
Conclusions
The post- and %ΔSUVmax of primary tumors were significantly associated with the pathological features and recurrence of ESCC under trimodal therapy. Therefore, FDG-PET can preoperatively predict the degree of aggressive tumor behavior in ESCC under trimodal therapy.

Similar content being viewed by others
References
Sjoquist KM, Burmeister BH, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011; 12: 681–692.
Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017; 390: 2383–96.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. CROSS Group: Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012; 366: 2074–84.
Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodal therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008; 26: 1086–92.
Sepesi B, Schmidt HE, Lada M, et al. Survival in patients with esophageal adenocarcinoma undergoing trimodality therapy is independent of regional lymph node location. Ann Thorac Surg. 2016; 101:1075–80.
Akutsu Y, Shuto K, Kono T, et al. The number of pathologic lymph nodes involved is still a significant prognostic factor even after neoadjuvant chemoradiotherapy in esophageal squamous cell carcinoma. J Surg Oncol. 2012; 105: 756–60.
Hamai Y, Hihara J, Emi M, et al. Evaluation of prognostic factors for esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy followed by surgery. World J Surg. 2018; 42: 1496–1505.
Hamai Y, Emi M, Ibuki Y, et al. Early recurrence and cancer death after trimodal therapy for esophageal squamous cell carcinoma. Anticancer Res. 2019; 39: 1433–1440.
Chen WH, Huang YL, Chao YK, et al. Prognostic significance of lymphovascular invasion in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2015; 22: 338–43.
Hamai Y, Hihara J, Taomoto J, Yamakita I, Ibuki Y, Okada M. Effects of neoadjuvant chemoradiotherapy on postoperative morbidity and mortality associated with esophageal cancer. Dis Esophagus. 2015; 28: 358–64.
Schmidt T, Lordick F, Herrmann K, Ott K. Value of functional imaging by PET in esophageal cancer. J Natl Compr Canc Netw. 2015; 13: 239–47.
Hamai Y, Hihara J, Emi M, et al. Ability of fluorine-18 fluorodeoxyglucose positron emission tomography to predict outcomes of neoadjuvant chemoradiotherapy followed by surgical treatment for esophageal squamous cell carcinoma. Ann Thorac Surg. 2016; 102: 1132–39.
Cong L, Wang S, Gao T, Hu L. The predictive value of 18F-FDG PET for pathological response of primary tumor in patients with esophageal cancer during or after neoadjuvant chemoradiotherapy: a meta-analysis. Jpn J Clin Oncol. 2016; 46:1118–26.
Sobin L, Gospodarowicz M, Wittekind C, eds. International Union Against Cancer (UICC): TNM classification of malignant tumours (7th edition). Wiley: New York, 2009.
Hamai Y, Hihara J, Emi M, et al. Results of neoadjuvant chemoradiotherapy with docetaxel and 5-fluorouracil followed by esophagectomy to treat locally advanced esophageal cancer. Ann Thorac Surg. 2015; 99: 1887–93.
Emi M, Hihara J, Hamai Y, et al. Neoadjuvant chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil for esophageal cancer. Cancer Chemother Pharmacol. 2012; 69: 1499–505.
Hamai Y, Hihara J, Emi M, et al. Effects of neoadjuvant chemoradiotherapy on pathological TNM Stage and their prognostic significance for surgically-treated esophageal squamous cell carcinoma. Anticancer Res. 2017; 37: 5639–46.
Murakami Y, Hamai Y, Emi M, et al. Long-term results of neoadjuvant chemoradiotherapy using cisplatin and 5-fluorouracil followed by esophagectomy for resectable, locally advanced esophageal squamous cell carcinoma. J Radiat Res. 2018; 59: 616–24.
Lee JR, Madsen MT, Bushnel D, Menda Y. A threshold method to improve standardized uptake value reproducibility. Nucl Med Commun. 2000; 21: 685–90.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000; 92: 205–16.
Beseth BD, Bedford R, Isacoff WH, Holmes EC, Cameron RB. Endoscopic ultrasound does not accurately assess pathologic stage of esophageal cancer after neoadjuvant chemoradiotherapy. Am Surg. 2000; 66: 827–31.
Schneider PM, Metzger R, Schaefer H, et al. Response evaluation by endoscopy, rebiopsy, and endoscopic ultrasound does not accurately predict histopathologic regression after neoadjuvant chemoradiation for esophageal cancer. Ann Surg. 2008; 248: 902–8.
Brücher BL, Stein HJ, Werner M, Siewert JR. Lymphatic vessel invasion is an independent prognostic factor in patients with a primary resected tumor with esophageal squamous cell carcinoma. Cancer. 2001; 92: 2228–33.
Zhu CM, Ling YH, ** SY, et al. Prognostic significance of the pN classification supplemented by vascular invasion for esophageal squamous cell carcinoma. PLoS One. 2014; 9: e96129.
Jeon JH, Lee JM, Moon DH, et al. Prognostic significance of venous invasion and maximum standardized uptake value of 18F-FDG PET/CT in surgically resected T1N0 esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017; 43: 471–7.
O JH, Jacene H, Luber B, Wang H, et al. Quantitation of cancer treatment response by 18F-FDG PET/CT: Multicenter assessment of measurement variability. J Nucl Med. 2017; 58: 1429–1434.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamai, Y., Emi, M., Ibuki, Y. et al. Predictions of Pathological Features and Recurrence Based on FDG-PET Findings of Esophageal Squamous Cell Carcinoma after Trimodal Therapy. Ann Surg Oncol 27, 4422–4430 (2020). https://doi.org/10.1245/s10434-020-08609-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08609-0