Abstract
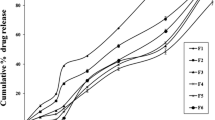
The rationale for the current investigation is to study the crude banana peel (CBP) powder efficiency as a novel natural time-dependent polymer along with a pH-sensitive polymer to develop flurbiprofen colon-specific tablets. The direct compression method is utilized to prepare the flurbiprofen-CBP matrix tablets using 9 mm punches on the rotary tableting machine and subsequently coated with Eudragit® S 100 by a dip coating method. The tablets were evaluated for various tableting properties and in vitro drug release studies. From the results of dissolution studies, the F6 formulation showed negligible drug release (5.76% in 5 h) in the upper gastrointestinal tract and progressive release in the colon (99.08% in 24 h). Mean dissolution time, T10%, and T80% were found to be 13.33 h, 5.8 h, and 20.7 h, respectively, which explains the efficiency of the present combination of polymers for colon-specific drug release. From the dissolution studies results of stability studies, the similarity index was calculated and found to be 74.75. In conclusion, utilizing CBP as a natural, time-dependent polymer in conjunction with Eudragit® S 100 to develop the flurbiprofen tablets seems like a promising approach for delivering drugs specifically to the colon.
Graphical Abstract






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
McCoubrey LE, Favaron A, Awad A, Orlu M, Gaisford S, Basit AW. Colonic drug delivery: Formulating the next generation of colon-targeted therapeutics. J Con Rel. 2023;353:1107–26.
Vemula SK, Veerareddy PR. Different approaches to design and evaluation of colon specific drug delivery systems. Int J Pharm Tech. 2009;1(1):1–35.
Doggwiler V, Lanz M, Paredes V, Lipps G, Imanidis G. Tablet formulation with dual control concept for efficient colonic drug delivery. Int J Pharm. 2023;631: 122499.
Kulkarni N, Jain P, Shindikar A, Suryawanshi P, Thorat N. Advances in the colon-targeted chitosan based drug delivery systems for the treatment of inflammatory bowel disease. Carbohydr Polym. 2022;14: 119351.
Veerareddy PR, Vemula SK. Formulation, evaluation and pharmacokinetics of colon targeted pulsatile system of flurbiprofen. J Drug Targ. 2012;20(8):703–14.
Vemula SK, Veerareddy PR. Development, evaluation and pharmacokinetics of time-dependent ketorolac tromethamine tablets. Exp Opin Drug Del. 2013;10(1):33–45.
Vemula SK. A novel approach to flurbiprofen pulsatile colonic release: Formulation and pharmacokinetics of double-compression-coated mini-tablets. AAPS PharmSciTech. 2015;16:1465–73.
Vemula SK. Formulation and pharmacokinetics of colon-specific double-compression coated mini-tablets: Chronopharmaceutical delivery of ketorolac tromethamine. Int J Pharm. 2015;491(1–2):35–41.
Sinha VR, Bhinge JR, Kumria R, Kumar M. Development of pulsatile systems for targeted drug delivery of celecoxib for prophylaxis of colorectal cancer. Drug Del. 2006;13(3):221–5.
Zaini HM, Roslan J, Saallah S, Munsu E, Sulaiman NS, Pindi W. Banana peels as a bioactive ingredient and its potential application in the food industry. J Functional Foods. 2022;92: 105054.
Zhou GJ, Li SH, Zhang YC, Fu YZ. Biosynthesis of CdS nanoparticles in banana peel extract. J Nanosci Nanotech. 2014;14(6):4437–42.
Lohmousavi SM, Abad HH, Noormohammadi G, Delkhosh B. Synthesis and characterization of a novel controlled release nitrogen-phosphorus fertilizer hybrid nanocomposite based on banana peel cellulose and layered double hydroxides nanosheets. Arab J Chem. 2020;13(9):6977–85.
Vemula SK, Veerareddy PR. Fast disintegrating tablets of flurbiprofen: Formulation and characterization. Lat Am J Pharm. 2011;30(6):1135–41.
Abbas FM, Saifullah R, Azhar ME. Assessment of physical properties of ripe banana flour prepared from two varieties: Cavendish and Dream banana. Int Food Res J. 2009;16(2)2):183–9.
Vemula SK. Colon specific drug delivery: Effect of Eudragit enteric coating on hydroxypropyl methylcellulose matrix tablets of flurbiprofen. J Young Pharmacists. 2015;7(4):373.
Verma A, Sharma B, Kalia S, Alsanie WF, Thakur S, Thakur VK. Carboxymethyl cellulose based sustainable hydrogel for colon-specific delivery of gentamicin. Int J Bio Macromol. 2023;228:773–82.
Dalei G, Das S, Jena SR, Jena D, Nayak J, Samanta L. In situ crosslinked dialdehyde guar gum-chitosan Schiff-base hydrogels for dual drug release in colorectal cancer therapy. Chem Eng Sci. 2023;269: 118482.
Matthews BR. Regulatory aspects of stability testing in Europe. Drug Dev Ind Pharm. 1999;25(7):831–56.
ICH Q1A. Stability testing guidelines: Stability testing of new drug substances and products. CPMP/ICH/380/95:1–13.
Staniforth JN, Aulton ME. Powder flow. Aulton’s pharmaceutics-the design and manufacture of medicines. 3rd ed. Churchill Livingstone: Elsevier; 2007. p. 168–79
Wu B, Shun N, Wei X, Wu W. Characterization of 5-fluorouracil release from hydroxypropylmethylcellulose compression-coated tablets. Pharm Dev Tech. 2007;12(2):203–10.
Vemula SK, Veerareddy PR, Devadasu VR. Pharmacokinetics of colon-specific pH and time-dependent flurbiprofen tablets. Eur J Drug Met Pharmacokinet. 2015;40:301–11.
Vemula SK, Bontha VK. Colon targeted guar gum compression coated tablets of flurbiprofen: Formulation, development, and pharmacokinetics. BioMed Res Int. 2013;2013:1–8. https://doi.org/10.1155/2013/287919.
Krishnaiah YS, Satyanarayana V, Kumar BD, Karthikeyan RS, Bhaskar P. In vivo pharmacokinetics in human volunteers: oral administered guar gum-based colon-targeted 5-fluorouracil tablets. Eur J Pharm Sci. 2003;19(5):355–62.
Wathoni N, Nguyen AN, Rusdin A, Umar AK, Mohammed AF, Motoyama K, Joni IM, Muchtaridi M. Enteric-coated strategies in colorectal cancer nanoparticle drug delivery system. Drug Design Dev Ther. 2020;21:4387–405.
Alderman DA. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int J Pharm Tech Prod Mfr. 1984;5(3):1–9.
Talukdar MM, Michoel A, Rombaut P, Kinget R. Comparative study on xanthan gum and hydroxypropyl methylcellulose as matrices for controlled-release drug delivery I. Compaction and in vitro drug release behaviour. Int J Pharm. 1996;129:233–41.
Wu B, Shun N, Wei X, Wu W. Characterization of 5-fluorouracil release from hydroxypropyl methylcellulose compression-coated tablets. Pharm Dev Tech. 2007;12(2):203–10.
Vemula SK, Veerareddy PR, Devadasu VR. Pharmacokinetics of ketorolac tromethamine compression-coated tablets for colon delivery. Drug Del Trans Res. 2014;4:310–9.
Krishnaiah YS, Satyanarayana S, Prasad YR, Rao SN. Evaluation of guar gum as a compression coat for drug targeting to colon. Int J Pharm. 1998;171(2):137–46.
Narala S, Nyavanandi D, Mandati P, Youssef AA, Alzahrani A, Kolimi P, Zhang F, Repka M. Preparation and in vitro evaluation of hot-melt extruded pectin-based pellets containing ketoprofen for colon targeting. Int J Pharm: X. 2023;5: 100156.
Dumpa NR, Sarabu S, Bandari S, Zhang F, Repka MA. Chronotherapeutic drug delivery of ketoprofen and ibuprofen for improved treatment of early morning stiffness in arthritis using hot-melt extrusion technology. AAPS PharmSciTech. 2018;19:2700–9.
Asghar LF, Chure CB, Chandran S. Colon specific delivery of indomethacin: Effect of incorporating pH sensitive polymers in xanthan gum matrix bases. AAPS PharmSciTech. 2009;10:418–29.
Acknowledgements
The authors acknowledge GITAM School of Pharmacy, Hyderabad, Telangana, India, and School of Pharmaceutical Sciences, Lovely Professional University, Jalandhar, Punjab, India, for providing chemicals and facilities for the successful completion of work.
Author information
Authors and Affiliations
Contributions
Sateesh Kumar Vemula and Bhaskar Daravath: formulation design and experimental work. Sridhar Babu Gummadi: concept and preparation of CBP powder. Sateesh Kumar Vemula and Bhaskar Daravath: writing—original draft. Michael Repka: review and editing.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vemula, S.K., Daravath, B., Gummadi, S.B. et al. Formulation and Development of Flurbiprofen Colon-Specific Eudragit Coated Matrix Tablets: Use of a Novel Crude Banana Peel Powder as a Time-Dependent Polymer. AAPS PharmSciTech 24, 189 (2023). https://doi.org/10.1208/s12249-023-02646-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02646-0