Abstract
Colorectal carcinoma (CRC) stands as a pressing global health issue, marked by the unbridled proliferation of immature cells influenced by multifaceted internal and external factors. Numerous studies have explored the intricate mechanisms of tumorigenesis in CRC, with a primary emphasis on signaling pathways, particularly those associated with growth factors and chemokines. However, the sheer diversity of molecular targets introduces complexity into the selection of targeted therapies, posing a significant challenge in achieving treatment precision. The quest for an effective CRC treatment is further complicated by the absence of pathological insights into the mutations or alterations occurring in tumor cells. This study reveals the transfer of signaling from the cell membrane to the nucleus, unveiling recent advancements in this crucial cellular process. By shedding light on this novel dimension, the research enhances our understanding of the molecular intricacies underlying CRC, providing a potential avenue for breakthroughs in targeted therapeutic strategies. In addition, the study comprehensively outlines the potential immune responses incited by the aberrant activation of signaling pathways, with a specific focus on immune cells, cytokines, and their collective impact on the dynamic landscape of drug development. This research not only contributes significantly to advancing CRC treatment and molecular medicine but also lays the groundwork for future breakthroughs and clinical trials, fostering optimism for improved outcomes and refined approaches in combating colorectal carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal carcinoma stands as a prominent contributor to cancer-related mortality on a global scale, and its intricate pathogenesis involves a multifaceted interplay of various factors. Genetic mutations, dysregulated signaling pathways, and compromised immune surveillance collectively contribute to the development and progression of this malignancy [1,2,3]. Many signaling pathways have been involved in the pathogenesis of colorectal carcinoma, that is so complex to understand the mechanisms. For example, the EGFR-mediated signaling pathway plays a crucial role in tumor cell proliferation, that influences cellular activities, such as nuclear transcription and mitochondrial metabolism [4,5,6]. In addition, hyperactivation of the Wnt signaling pathway is observed in most CRC cases. Approximately 85% of these cases exhibit loss of APC function, leading to destabilization of free β-catenin [7]. CXCLs and their cognate receptors are also implicated in mediating tumor growth and metastasis [8]. Notch, typically involved in maintaining progenitor/stem cell populations, is found to be constitutively activated in colon cancers [9]. Additionally, the transcription factor STAT3 plays a significant role in forming the tumor microenvironment [10]. Transmembrane receptors, such as JMJD6 and AXL, are associated with the proliferation and motility of tumor cells [11, 12]. So many signaling pathways have confused clinicians and physicians, that commanded a brief review about this.
Various agents targeting these molecules have been developed, including traditional Chinese medicines and small molecule inhibitors, with clinical trials underway to evaluate their efficacy. The heterogeneity of CRC broadens the scope for drug development, though it complicates the selection of the appropriate therapy. Organoids, derived from human induced pluripotent stem cells (iPSCs) or human embryonic stem cells (hESCs), has been considered as a good tool for drug screening in colorectal carcinoma patients recently [13]. However, In the quest for personalized treatment, cost-effectiveness analysis is emerging as a crucial facet, optimizing therapy selection for individual patients [14, 15]. As clinical trials progress and our understanding deepens, the intricate interplay of molecular pathways in CRC becomes increasingly discernible, paving the way for refined, tailored interventions and fostering hope for improved outcomes in the battle against colorectal carcinoma.
Current standard treatment
The pivotal role of effective first-line therapy in determining colorectal carcinoma (CRC) outcomes cannot be overstated, as clinicians navigate through a myriad of factors that shape their choices in clinical decision-making. The location of the tumor within the colorectum emerges as a crucial factor influencing therapeutic decisions [16,17,18,19]. Notably, patients with left-sided tumors stand to gain significant benefits from certain treatment modalities. This observation underscores the importance of tailoring therapies based on tumor location to optimize outcomes. Conversely, the scenario differs for patients with right-sided tumors, where the efficacy of initial epidermal growth factor receptor (EGFR)-based therapy may be limited. Beyond the initial response rate, the benefits of such therapies may not be as pronounced in this subgroup of patients. This nuanced understanding of the varying responses based on tumor location adds a layer of complexity to the selection of first-line therapies for CRC. It emphasizes the need for personalized treatment strategies that consider the specific characteristics of the tumor, ultimately aiming to maximize the effectiveness of therapeutic interventions and improve overall outcomes for individuals facing colorectal carcinoma. A worse prognosis for OS, PFS, and ORR has been observed in patients with right-sided tumor compared with those with left-sided tumor. The determination of second-line treatment hinges on the outcomes and responses observed during the administration of first-line systemic therapies, a pivotal step in the sequential management of colorectal carcinoma. Notably, anti-angiogenic agents, such as bevacizumab, emerge as frequently employed components in the second-line treatment regimen [20]. These agents, designed to inhibit angiogenesis and disrupt the tumor's blood supply, play a crucial role in extending the spectrum of therapeutic options available for patients post-first-line intervention. The utilization of anti-angiogenic agents underscores the strategic consideration of targeted therapies that address specific aspects of tumor progression, contributing to a comprehensive and personalized approach in the ongoing battle against colorectal carcinoma. The selection and customization of third-line treatments are intricately tied to the identification of specific molecular markers, thereby tailoring therapeutic approaches to the unique characteristics of the colorectal carcinoma (CRC). In cases where CRC exhibits a wild-type RAS status, the emphasis is often on utilizing kinase inhibitors designed to target and modulate specific molecular pathways associated with tumor progression [21]. This tailored strategy acknowledges the importance of aligning treatment with the molecular profile of the tumor, optimizing the chances of therapeutic success. Furthermore, in CRC cases characterized by high microsatellite instability (MSI), third-line interventions often incorporate immunotherapy as a key component. Agents such as pembrolizumab, nivolumab, or ipilimumab, known for their immune checkpoint inhibitory properties, are strategically employed to leverage the body's immune system against the tumor. This personalized approach, based on the presence of specific molecular markers, not only reflects the evolving landscape of precision medicine but also underscores the importance of leveraging targeted therapies and immunomodulation for enhanced efficacy in the management of advanced colorectal carcinoma.
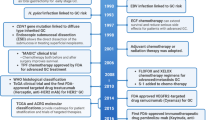
The therapeutic landscape for metastatic colorectal carcinoma (mCRC) has evolved, and in 2004, a notable recommendation emerged for the management of mCRC resistant to irinotecan-based therapy. Specifically, the combination of an epidermal growth factor receptor (EGFR) antibody with irinotecan was endorsed as a strategic intervention for cases where the tumor expresses EGFR. This combination therapy represented a significant advancement, introducing a targeted approach to address resistance issues observed with irinotecan-based regimens [22]. The landscape of metastatic colorectal carcinoma (mCRC) witnessed a significant development in 2006 with the approval of panitumumab, a fully human IgG2 monoclonal antibody, for third-line treatment in cases where the tumor expresses epidermal growth factor receptor (EGFR) [23]. This milestone marked a substantial stride in the quest for effective and targeted therapies tailored to the molecular characteristics of mCRC. Panitumumab might offer a more effective long-term treatment option for mCRC patients compared to cetuximab [24, 25]. Unfortunately, intrinsic and extrinsic resistance limits its use in colorectal cancer. Various strategies, including new EGFR-targeted inhibitors, combination therapies, novel cytotoxic drugs, and metabolic regulators, have been employed to overcome this resistance [26]. Bevacizumab, a humanized anti-VEGF monoclonal antibody, demonstrated promising preclinical and clinical efficacy against CRC, especially when combined with chemotherapy, leading to its approval in 2004 [27]. To counter the resistance of bevacizumab, targeting tumor cell-derived CCL2, a candidate gene involved in ETV5 + colorectal cancer, has been identified. The combination of bevacizumab with an anti-CCL2 antibody has shown a synergistic effect in inhibiting tumor growth and angiogenesis [28].
The effectiveness of EGFR (epidermal growth factor receptor) and VEGFR (vascular endothelial growth factor receptor) inhibitors faces limitations in KRAS wild-type colorectal carcinoma (CRC). In cases where the KRAS gene is wild-type, the use of inhibitors targeting EGFR and VEGFR pathways has demonstrated a certain level of efficacy. However, it is essential to acknowledge that neither cetuximab (an EGFR inhibitor) nor bevacizumab (a VEGFR inhibitor) has exhibited superior efficacy in the context of first-line treatment among patients with KRAS-mutated metastatic colorectal carcinoma (mCRC) [29], while combinations such as EGFR inhibitors with PARP, which is a family of proteins involved in a number of cellular process, such as DNA repair and genomic stability, inhibitors (olaparib) have effectively curbed tumor growth in KRAS-mutant cancer cell-derived xenograft models [30]. Vigilant consideration of adverse effects is paramount in the comprehensive evaluation of treatment options for colorectal carcinoma. Notably, the combination of XELIRI (capecitabine plus irinotecan) with bevacizumab, a regimen that has demonstrated promising response rates, demands careful attention due to the prevalence of certain side effects, most notably neutropenia, in patients undergoing this treatment [31]. Clinical evidence has demonstrated that pegfilgrastim plays a pivotal role in diminishing the incidence of grade 3/4 febrile neutropenia (FN). Pegfilgrastim, a form of granulocyte colony-stimulating factor (G-CSF), stands out as a proactive therapeutic intervention designed to address the heightened risk of FN, particularly in individuals undergoing treatments associated with a high risk of neutropenia. [32].
EGFR mediated signaling transduction
Aberrant activation of the epidermal growth factor receptor (EGFR), characterized by dysregulated cascade reactions, plays a pivotal role in the uncontrolled formation of tumor masses and the release of cytokines. This aberration in EGFR signaling pathways provides a crucial window of opportunity for disease control, making EGFR antibodies a cornerstone in the standard therapy for colorectal carcinoma (CRC) patients. However, the persistent challenge of resistance has restricted the widespread application of EGFR antibodies over the years. In response to resistance mechanisms, a paradigm shift in therapeutic strategies has been witnessed. The introduction of ATP-competitive and covalent binding inhibitors is aimed at targeting downstream molecules within the intricate signaling network, including RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathways. By addressing these downstream components, therapeutic interventions seek to overcome resistance and re-establish control over the tumor's growth and survival mechanisms. Understanding the intricate biological effects of these pathways assumes considerable significance in the quest to enhance the efficacy of clinical treatment for CRC. Insights into the crosstalk and interplay within these signaling cascades provide a foundation for develo** targeted therapies that can navigate around resistance mechanisms. This elucidation becomes a cornerstone in refining treatment strategies, offering the potential for more effective interventions and improved outcomes in the dynamic landscape of colorectal carcinoma management.
RAS-RAF-MEK-ERK implications in colon cancer
The downstream signaling pathway, RAS-RAF-MEK-ERK, constitutes a crucial component of the extensive serine-threonine kinase family, exerting profound influence on various aspects of cellular behavior. This intricate cascade plays a multifaceted role in key cellular processes, including cell proliferation, metastasis, angiogenesis, and extracellular matrix degradation [33]. EGFR, also known as ERBB, a glycoprotein with intracellular tyrosine kinase and extracellular protein ligands, but the ERBB receptors are subfamily of four tyrosine kinase receptors including EGFR, HER2/neu, HER3, and HER4 (https://www.ncbi.nlm.nih.gov/books/NBK482459/). Upon the binding of epidermal growth factor (EGF) to the epidermal growth factor receptor (EGFR), a cascade of molecular events is initiated at the membrane's inner face. This intricate process involves the recruitment of growth-factor-receptor bound protein 2 (GRB2) and son of sevenless (SOS) to the membrane, marking a critical step in the activation of the GTP-binding protein RAS. This molecular signaling pathway plays a pivotal role in transducing extracellular signals into the cell, ultimately influencing key cellular processes. In recent years, there has been a substantial expansion in our understanding of the intricacies of this signaling pathway, particularly in the context of colorectal carcinoma (CRC). The molecular events triggered by EGFR activation and the subsequent activation of RAS have been scrutinized with increased precision. This heightened comprehension extends beyond the basic mechanics of the pathway and delves into the specific alterations and dysregulations that characterize colorectal cancer [34]. Aberrant activations within cellular signaling pathways have been identified as key contributors to pathological conditions, and this includes dysregulation in the epidermal growth factor receptor (EGFR), RAS, and BRAF. The intricate interplay of these components often leads to abnormal signaling cascades within the cell. In particular, the downstream effector, extracellular signal-regulated kinase (ERK), has been implicated in aberrant activation, representing a significant facet of these perturbed signaling pathways [35]. The influence of aberrant activations, including EGFR, RAS, and BRAF, extends beyond the initial signaling events to impact downstream molecules crucial for cellular regulation. Notably, downstream effectors such as cyclin D1, MMP1, P53, and c-MYC are intricately influenced by these dysregulated signaling pathways [36].
Mutations in the RAS gene family, encompassing KRAS, HRAS, and NRAS, represent a prevalent genetic alteration in colorectal carcinoma (CRC). Among these, KRAS mutations are particularly noteworthy, being identified in over 30% of CRC patients. Within KRAS mutations, there is a notable association with malignancy development, especially when occurring in codon 12 [37]. Another noteworthy mutation in colorectal carcinoma (CRC) involves the BRAF gene, which serves as a downstream molecule in the RAS signaling pathway. The mutation in BRAF represents a significant alteration and is among the most prevalent mutations observed in CRC [38]. While RAF is recruited by activated RAS, the activation mechanism of BRAF differs significantly, primarily taking place through a distinctive process involving dimerization with various forms of RAF. Unlike the straightforward activation process seen in other RAF family members, BRAF activation is intricately linked to the formation of dimers, representing a unique regulatory mechanism within the RAS-RAF-MEK-ERK signaling pathway [39]. MEK and ERK, downstream of RAF, are activated by RAF through phosphorylation, leading to the activation of ERK, mitogen-activated protein kinase (MAPK). Activated ERK regulates gene expression in the nucleus with the aid of transcription factors, including c-FOS, c-JUN, S6, and others. The signaling transduction is more complex than described, involving intricate pathways like MAPK, which includes ERK1/2, JNK, and P38. The function of c-FOS can be regulated by both JNK and ERK, occasionally by H-RAS [40].
Despite the development of EGFR antibodies, such as cetuximab and panitumumab, as targeted therapies for colorectal carcinoma (CRC), the clinical outcomes have presented challenges. The disease has shown progression in over 50% of patients treated with these EGFR antibodies, indicating the complexity of overcoming resistance mechanisms in CRC. KRAS mutations, found in 40–50% of patients, unsurprisingly diminished cetuximab's efficacy [41, 42]. RAS, a GTP-binding protein, has long presented a formidable challenge in therapeutic targeting due to its elusive nature. Despite extensive efforts, identifying effective targets for RAS inhibition has proven to be a complex task. However, recent advancements have seen the development of several inhibitors specifically designed to target a distinct mutation within the KRAS gene known as KRASG12C. The synergistic potential of combining anti-BRAF and anti-EGFR therapies has demonstrated noteworthy effectiveness in the management of metastatic colorectal carcinoma (mCRC) patients harboring the BRAFV600E mutation, particularly those who have undergone prior treatment with an anti-EGFR inhibitor [43]. Encorafenib combined with cetuximab is reported as the new standard care for BRAFV600E mCRC [44, 45]. Adding an MEK inhibitor to this regimen does not extend survival but increases the objective response rate (ORR) and toxicity. A recent study reveals that a chimeric receptor, CD16-158-valine on T cells, overcomes cetuximab resistance in KRAS mutant CRC, which indicates a new direction of CRC treatment, the combination of immune and molecule targets [46]. Other clinical trials have been summarized (Fig. 1), that phase 3 clinical trials have been activated in RAS inhibitors while those has been terminated in MEK and RAF inhibitors. Clinical trials in ERK inhibitors have limited in phase 1 clinical trials, which verified the safety but command much attentions.
PI3K-AKT-mTOR is involved in tumor cell growth, proliferation, and differentiation
PI3K/Akt/mTOR, are crucial kinases that control essential functions of cells. Various biological molecules, such as epidermal growth factors, sonic hedgehog, IGF-1, insulin, and calmodulin, activate this signaling pathway. Other molecules antagonize this pathway, including phosphatase and tensin homolog (PTEN), glycogen synthase kinase 3β, and transcription factor HB9 [125], additional chemokines such as CXCL14, CXCL3, and CXCL12 serve as predictive factors [126,127,128]. In CRC metastasis, two distinct CXCL12 pathways are identified: CXCL12-CXCR4 and CXCL12-CXCR7 axes, with CXCL directly influencing metastasis through the Wnt-β-catenin signaling pathway [129].
Targeting CXCR, specifically the frizzled transmembrane Ca2 + signaling pathway, demonstrates significant potential in CRC treatment, encompassing CCR1, CCR5, and CXCR2 (Fig. 4). CCR1 antagonists curb CRC progression by targeting CCR1-expressing myeloid cells [130]. CCR5 shows high expression in both primary and liver metastatic masses, associated with a reduced cytotoxic/regulatory T cell ratio and diminished M2 macrophage polarization [131]. Maraviroc, a CCR5 inhibitor, curtails T cell recruitment to colorectal carcinoma and hinders liver metastasis in advanced refractory CRC [132, 133]. The safety and efficacy of pembrolizumab and maraviroc have been explored in phase 1 clinical trial (NCT03274804) [134]. A phase 2 clinical trial is investigating the combination effects of Navarixin, a CXCL1/2 inhibitor, with pembrolizumab in advanced microsatellite CRC (NCT03473925) [135]. Although many targeted inhibitors are used for treating hematological diseases, no CCR or CXCL inhibitors have been have been approved by FDA yet.
Notch-related cell–cell contacts and signaling pathways
The Notch signaling pathway, a key cellular communication system, regulates cell proliferation, differentiation, and apoptosis [136,137,138]. This pathway comprises Notch receptors (Notch1-4), ligands (Jagged1-2, DLL1, DLL3, DLL4), and numerous downstream genes such as p21 and hes-1 [139]. In healthy colonic tissues, Notch signaling plays an essential role in maintaining intestinal epithelial integrity [140]. Immunohistochemistry studies reveal elevated Notch1 and Jagged1 expression in colorectal carcinoma and adenoma compared to adjacent carcinoma and normal tissues [ Not applicable. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointestinal Cancer Res. 2012;5(1):19–27. Koveitypour Z, Panahi F, Vakilian M, Peymani M, Seyed Forootan F, Nasr Esfahani MH, et al. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9:97. https://doi.org/10.1186/s13578-019-0361-4. Parcesepe P, Giordano G, Laudanna C, Febbraro A, Pancione M. Cancer-associated immune resistance and evasion of immune surveillance in colorectal cancer. Gastroenterol Res Pract. 2016;2016:6261721. https://doi.org/10.1155/2016/6261721. Krasinskas AM. EGFR signaling in colorectal carcinoma. Pathol Res Int. 2011;2011: 932932. https://doi.org/10.4061/2011/932932. Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12(1):3–20. https://doi.org/10.1002/1878-0261.12155. Shimizu S, Kondo J, Onuma K, Coppo R, Ota K, Kamada M, et al. Inhibition of the bone morphogenetic protein pathway suppresses tumor growth through downregulation of epidermal growth factor receptor in MEK/ERK-dependent colorectal cancer. Cancer Sci. 2023;114(9):3636–48. https://doi.org/10.1111/cas.15882. Schatoff EM, Leach BI, Dow LE. Wnt signaling and colorectal cancer. Curr Colorectal Cancer Reports. 2017;13(2):101–10. https://doi.org/10.1007/s11888-017-0354-9. Łukaszewicz-Zając M, Pączek S, Mroczko P, Kulczyńska-Przybik A. The significance of CXCL1 and CXCL8 as well as their specific receptors in colorectal cancer. Cancer Manage Res. 2020;12:8435–43. https://doi.org/10.2147/cmar.S267176. Qiao L, Wong BC. Role of Notch signaling in colorectal cancer. Carcinogenesis. 2009;30(12):1979–86. https://doi.org/10.1093/carcin/bgp236. Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4(3):176–85. https://doi.org/10.1007/s13238-013-2084-3. Wang F, He L, Huangyang P, Liang J, Si W, Yan R, et al. JMJD6 promotes colon carcinogenesis through negative regulation of p53 by hydroxylation. Plos Biol. 2014;12(3):e1001819. https://doi.org/10.1371/journal.pbio.1001819. Colavito SA. AXL as a target in breast cancer therapy. J Oncol. 2020;2020:5291952. https://doi.org/10.1155/2020/5291952. Mertens S, Huismans MA, Verissimo CS, Ponsioen B, Overmeer R, Proost N, et al. Drug-repurposing screen on patient-derived organoids identifies therapy-induced vulnerability in KRAS-mutant colon cancer. Cell Rep. 2023;42(4):112324. https://doi.org/10.1016/j.celrep.2023.112324. Gheysariyeha F, Rahimi F, Tabesh E, Hemami MR, Adibi P, Rezayatmand R. Cost-effectiveness of colorectal cancer screening strategies: a systematic review. Eur J Cancer Care. 2022;31(6):e13673. https://doi.org/10.1111/ecc.13673. Ran T, Cheng CY, Misselwitz B, Brenner H, Ubels J, Schlander M. Cost-effectiveness of colorectal cancer screening strategies-a systematic review. Clin Gastroenterol Hepatol. 2019;17(10):1969–81.e15. https://doi.org/10.1016/j.cgh.2019.01.014. Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713–29. https://doi.org/10.1093/annonc/mdx175. Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98. https://doi.org/10.1016/j.ejca.2016.10.007. Aljehani MA, Morgan JW, Guthrie LA, Jabo B, Ramadan M, Bahjri K, et al. Association of primary tumor site with mortality in patients receiving Bevacizumab and Cetuximab for metastatic colorectal cancer. JAMA Surg. 2018;153(1):60–7. https://doi.org/10.1001/jamasurg.2017.3466. Wong HL, Lee B, Field K, Lomax A, Tacey M, Shapiro J, et al. Impact of primary tumor site on Bevacizumab efficacy in metastatic colorectal cancer. Clin Colorectal Cancer. 2016;15(2):e9–15. https://doi.org/10.1016/j.clcc.2016.02.007. Bennouna J, Hiret S, Bertaut A, Bouché O, Deplanque G, Borel C, et al. Continuation of Bevacizumab vs Cetuximab plus chemotherapy after first progression in KRAS wild-type metastatic colorectal cancer: The UNICANCER PRODIGE18 randomized clinical trial. JAMA Oncol. 2019;5(1):83–90. https://doi.org/10.1001/jamaoncol.2018.4465. Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer. 2019;109:70–83. https://doi.org/10.1016/j.ejca.2018.12.019. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45. https://doi.org/10.1056/NEJMoa033025. Saltz L, Easley C, Kirkpatrick P. Panitumumab. Nat Rev Drug Discovery. 2006;5(12):987–8. https://doi.org/10.1038/nrd2204. Nielsen DL, Pfeiffer P, Jensen BV. Six cases of treatment with panitumumab in patients with severe hypersensitivity reactions to cetuximab. Ann Oncol. 2009;20(4):798. https://doi.org/10.1093/annonc/mdp040. Seront E, Marot L, Coche E, Gala JL, Sempoux C, Humblet Y. Successful long-term management of a patient with late-stage metastatic colorectal cancer treated with panitumumab. Cancer Treat Rev. 2010;36(Suppl 1):S11–4. https://doi.org/10.1016/s0305-7372(10)70002-5. Zhou J, Ji Q, Li Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: underlying mechanisms and reversal strategies. J Exp Clin Cancer Res. 2021;40(1):328. https://doi.org/10.1186/s13046-021-02130-2. New treatments for colorectal cancer. FDA Consum. 2004;38(3):17. https://permanent.access.gpo.gov/lps1609/www.fda.gov/fdac/features/2004/304_cancer.html. Feng H, Liu K, Shen X, Liang J, Wang C, Qiu W, et al. Targeting tumor cell-derived CCL2 as a strategy to overcome Bevacizumab resistance in ETV5(+) colorectal cancer. Cell Death Dis. 2020;11(10):916. https://doi.org/10.1038/s41419-020-03111-7. Stintzing S, von FischerWeikersthal L, Decker T, Vehling-Kaiser U, Jäger E, Heintges T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol. 2012;23(7):1693–9. https://doi.org/10.1093/annonc/mdr571. Zhong L, Wang R, Wang Y, Peng S, Ma Y, Ding S, et al. Dual inhibition of VEGF and PARP suppresses KRAS-mutant colorectal cancer. Neoplasia (New York, NY). 2020;22(9):365–75. https://doi.org/10.1016/j.neo.2020.06.001. Ando K, Emi Y, Suenaga T, Hamanoue M, Maekawa S, Sakamoto Y, et al. A prospective study of XELIRI plus bevacizumab as a first-line therapy in Japanese patients with unresectable or recurrent colorectal cancer (KSCC1101). Int J Clin Oncol. 2017;22(5):913–20. https://doi.org/10.1007/s10147-017-1140-z. Pinter T, Klippel Z, Cesas A, Croitoru A, Decaestecker J, Gibbs P, et al. A phase III, randomized, double-blind, placebo-controlled trial of Pegfilgrastim in patients receiving first-line FOLFOX/Bevacizumab or FOLFIRI/Bevacizumab for locally advanced or metastatic colorectal cancer: final results of the Pegfilgrastim and Anti-VEGF Evaluation Study (PAVES). Clin Colorectal Cancer. 2017;16(2):103–14.e3. https://doi.org/10.1016/j.clcc.2016.08.008. Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007. https://doi.org/10.3892/etm.2020.8454. Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6(5):322–7. https://doi.org/10.1016/s1470-2045(05)70168-6. Müller MF, Ibrahim AE, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Archiv. 2016;469(2):125–34. https://doi.org/10.1007/s00428-016-1956-3. Park S, Jung HH, Park YH, Ahn JS, Im YH. ERK/MAPK pathways play critical roles in EGFR ligands-induced MMP1 expression. Biochem Biophys Res Commun. 2011;407(4):680–6. https://doi.org/10.1016/j.bbrc.2011.03.075. Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327(6120):293–7. https://doi.org/10.1038/327293a0. Nandan MO, Yang VW. An update on the biology of RAS/RAF mutations in colorectal cancer. Curr Colorectal Cancer Rep. 2011;7(2):113–20. https://doi.org/10.1007/s11888-011-0086-1. Zhang M, Maloney R, Jang H, Nussinov R. The mechanism of Raf activation through dimerization. Chem Sci. 2021;12(47):15609–19. https://doi.org/10.1039/d1sc03444h. Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature. 1994;371(6493):171–5. https://doi.org/10.1038/371171a0. Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–65. https://doi.org/10.1056/NEJMoa0804385. Benhattar J, Losi L, Chaubert P, Givel JC, Costa J. Prognostic significance of K-ras mutations in colorectal carcinoma. Gastroenterology. 1993;104(4):1044–8. https://doi.org/10.1016/0016-5085(93)90272-e. Hafliger E, Boccaccino A, Lapeyre-Prost A, Perret A, Gallois C, Antista M, et al. Encorafenib plus cetuximab treatment in BRAF V600E-mutated metastatic colorectal cancer patients pre-treated with an anti-EGFR: An AGEO-GONO case series. Eur J Cancer. 2022;168:34–40. https://doi.org/10.1016/j.ejca.2022.03.011. Kopetz S, Guthrie KA, Morris VK, Lenz HJ, Magliocco AM, Maru D, et al. Randomized trial of Irinotecan and Cetuximab with or without Vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol. 2021;39(4):285–94. https://doi.org/10.1200/jco.20.01994. Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, et al. Encorafenib plus Cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39(4):273–84. https://doi.org/10.1200/jco.20.02088. Arriga R, Caratelli S, Lanzilli G, Ottaviani A, Cenciarelli C, Sconocchia T, et al. CD16-158-valine chimeric receptor T cells overcome the resistance of KRAS-mutated colorectal carcinoma cells to cetuximab. Int J Cancer. 2020;146(9):2531–8. https://doi.org/10.1002/ijc.32618. **e Y, Shi X, Sheng K, Han G, Li W, Zhao Q, et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol Med Rep. 2019;19(2):783–91. https://doi.org/10.3892/mmr.2018.9713. Ediriweera MK, Tennekoon KH, Samarakoon SR. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin Cancer Biol. 2019;59:147–60. https://doi.org/10.1016/j.semcancer.2019.05.012. Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. https://doi.org/10.3389/fnmol.2011.00051. Narayanankutty A. PI3K/ Akt/ mTOR pathway as a therapeutic target for colorectal cancer: a review of preclinical and clinical evidence. Curr Drug Targets. 2019;20(12):1217–26. https://doi.org/10.2174/1389450120666190618123846. Jiang T, Wang H, Liu L, Song H, Zhang Y, Wang J, et al. CircIL4R activates the PI3K/AKT signaling pathway via the miR-761/TRIM29/PHLPP1 axis and promotes proliferation and metastasis in colorectal cancer. Mol Cancer. 2021;20(1):167. https://doi.org/10.1186/s12943-021-01474-9. Bishnupuri KS, Alvarado DM, Khouri AN, Shabsovich M, Chen B, Dieckgraefe BK, et al. IDO1 and Kynurenine pathway metabolites activate PI3K-Akt signaling in the neoplastic colon epithelium to promote cancer cell proliferation and inhibit apoptosis. Can Res. 2019;79(6):1138–50. https://doi.org/10.1158/0008-5472.can-18-0668. Duan S, Huang W, Liu X, Liu X, Chen N, Xu Q, et al. IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways. J Exp Clin Cancer Res. 2018;37(1):304. https://doi.org/10.1186/s13046-018-0980-3. Bender A, Opel D, Naumann I, Kappler R, Friedman L, von Schweinitz D, et al. PI3K inhibitors prime neuroblastoma cells for chemotherapy by shifting the balance towards pro-apoptotic Bcl-2 proteins and enhanced mitochondrial apoptosis. Oncogene. 2011;30(4):494–503. https://doi.org/10.1038/onc.2010.429. Fokas E, Im JH, Hill S, Yameen S, Stratford M, Beech J, et al. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature. Can Res. 2012;72(1):239–48. https://doi.org/10.1158/0008-5472.can-11-2263. Kim IA, Bae SS, Fernandes A, Wu J, Muschel RJ, McKenna WG, et al. Selective inhibition of Ras, phosphoinositide 3 kinase, and Akt isoforms increases the radiosensitivity of human carcinoma cell lines. Can Res. 2005;65(17):7902–10. https://doi.org/10.1158/0008-5472.can-05-0513. LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34(31):3803–15. https://doi.org/10.1200/jco.2014.59.0018. Wang J, Kuropatwinski K, Hauser J, Rossi MR, Zhou Y, Conway A, et al. Colon carcinoma cells harboring PIK3CA mutations display resistance to growth factor deprivation induced apoptosis. Mol Cancer Ther. 2007;6(3):1143–50. https://doi.org/10.1158/1535-7163.mct-06-0555. Deng J, Bai X, Feng X, Ni J, Beretov J, Graham P, et al. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer. 2019;19(1):618. https://doi.org/10.1186/s12885-019-5824-9. Blair HA. Duvelisib: first global approval. Drugs. 2018;78(17):1847–53. https://doi.org/10.1007/s40265-018-1013-4. Dhillon S, Keam SJ. Umbralisib: first approval. Drugs. 2021;81(7):857–66. https://doi.org/10.1007/s40265-021-01504-2. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–40. https://doi.org/10.1056/NEJMoa1813904. Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. https://doi.org/10.1186/s12943-019-0954-x. Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15(5):273–91. https://doi.org/10.1038/nrclinonc.2018.28. Idelalisib. LiverTox: clinical and research information on drug-induced liver injury. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30(3):282–90. https://doi.org/10.1200/jco.2011.36.1360. Rodon J, Braña I, Siu LL, De Jonge MJ, Homji N, Mills D, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32(4):670–81. https://doi.org/10.1007/s10637-014-0082-9. Wu YF, Li ZY, Dong LL, Li WJ, Wu YP, Wang J, et al. Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation. Autophagy. 2020;16(3):435–50. https://doi.org/10.1080/15548627.2019.1628536. Goodwin R, Jonker D, Chen E, Kennecke H, Cabanero M, Tsao MS, et al. A phase Ib study of a PI3Kinase inhibitor BKM120 in combination with panitumumab in patients with KRAS wild-type advanced colorectal cancer. Invest New Drugs. 2020;38(4):1077–84. https://doi.org/10.1007/s10637-019-00814-3. Wang Z, Zhou H, Zheng H, Zhou X, Shen G, Teng X, et al. Autophagy-based unconventional secretion of HMGB1 by keratinocytes plays a pivotal role in psoriatic skin inflammation. Autophagy. 2021;17(2):529–52. https://doi.org/10.1080/15548627.2020.1725381. Kim S, Lee JY, Shin SG, Kim JK, Silwal P, Kim YJ, et al. ESRRA (estrogen related receptor alpha) is a critical regulator of intestinal homeostasis through activation of autophagic flux via gut microbiota. Autophagy. 2021;17(10):2856–75. https://doi.org/10.1080/15548627.2020.1847460. Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. 2022;80:1–17. https://doi.org/10.1016/j.semcancer.2019.12.008. Cui X, Qian DW, Jiang S, Shang EX, Zhu ZH, Duan JA. Scutellariae Radix and Coptidis Rhizoma improve glucose and lipid metabolism in T2DM Rats via regulation of the metabolic profiling and MAPK/PI3K/Akt signaling pathway. Int J Mol Sci. 2018;19(11):3634. https://doi.org/10.3390/ijms19113634. Sun X, Ng TTH, Sham KWY, Zhang L, Chan MTV, Wu WKK, et al. Bufalin, a traditional Chinese medicine compound, prevents tumor formation in two murine models of colorectal cancer. Cancer Prev Res (Phila). 2019;12(10):653–66. https://doi.org/10.1158/1940-6207.capr-19-0134. Reidy DL, Vakiani E, Fakih MG, Saif MW, Hecht JR, Goodman-Davis N, et al. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28(27):4240–6. https://doi.org/10.1200/jco.2010.30.4154. Martinelli E, Troiani T, Morgillo F, Rodolico G, Vitagliano D, Morelli MP, et al. Synergistic antitumor activity of sorafenib in combination with epidermal growth factor receptor inhibitors in colorectal and lung cancer cells. Clin Cancer Res. 2010;16(20):4990–5001. https://doi.org/10.1158/1078-0432.ccr-10-0923. Galizia G, Lieto E, De Vita F, Orditura M, Castellano P, Troiani T, et al. Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26(25):3654–60. https://doi.org/10.1038/sj.onc.1210381. Stremitzer S, Sebio A, Stintzing S, Lenz HJ. Panitumumab safety for treating colorectal cancer. Expert Opin Drug Saf. 2014;13(6):843–51. https://doi.org/10.1517/14740338.2014.915024. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8(4):303–6. https://doi.org/10.1634/theoncologist.8-4-303. Pagán B, Isidro AA, Cruz ML, Ren Y, Coppola D, Wu J, et al. Erlotinib inhibits progression to dysplasia in a colitis-associated colon cancer model. World J Gastroenterol. 2011;17(44):4858–66. https://doi.org/10.3748/wjg.v17.i44.4858. Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Investig. 2014;124(6):2668–82. https://doi.org/10.1172/jci73014. Élez E, Kocáková I, Höhler T, Martens UM, Bokemeyer C, Van Cutsem E, et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann Oncol. 2015;26(1):132–40. https://doi.org/10.1093/annonc/mdu474. Sclafani F, Kim TY, Cunningham D, Kim TW, Tabernero J, Schmoll HJ, et al. A randomized phase II/III study of Dalotuzumab in combination with Cetuximab and Irinotecan in Chemorefractory, KRAS wild-type, metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(12):djv258. https://doi.org/10.1093/jnci/djv258. Lee PC, Chiou YC, Wong JM, Peng CL, Shieh MJ. Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody. Biomaterials. 2013;34(34):8756–65. https://doi.org/10.1016/j.biomaterials.2013.07.067. Zalba S, Contreras AM, Haeri A, Ten Hagen TL, Navarro I, Koning G, et al. Cetuximab-oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J Contr Rel. 2015;210:26–38. https://doi.org/10.1016/j.jconrel.2015.05.271. Yoon J, Koo KH, Choi KY. MEK1/2 inhibitors AS703026 and AZD6244 may be potential therapies for KRAS mutated colorectal cancer that is resistant to EGFR monoclonal antibody therapy. Can Res. 2011;71(2):445–53. https://doi.org/10.1158/0008-5472.can-10-3058. Misale S, Arena S, Lamba S, Siravegna G, Lallo A, Hobor S, et al. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med. 2014;6(224):224ra26. https://doi.org/10.1126/scitranslmed.3007947. Troiani T, Napolitano S, Martini G, Martinelli E, Cardone C, Normanno N, et al. Maintenance treatment with Cetuximab and BAY86-9766 increases antitumor efficacy of Irinotecan plus Cetuximab in human colorectal cancer Xenograft models. Clin Cancer Res. 2015;21(18):4153–64. https://doi.org/10.1158/1078-0432.ccr-15-0211. Troiani T, Napolitano S, Vitagliano D, Morgillo F, Capasso A, Sforza V, et al. Primary and acquired resistance of colorectal cancer cells to anti-EGFR antibodies converge on MEK/ERK pathway activation and can be overcome by combined MEK/EGFR inhibition. Clin Cancer Res. 2014;20(14):3775–86. https://doi.org/10.1158/1078-0432.ccr-13-2181. Rosa R, Melisi D, Damiano V, Bianco R, Garofalo S, Gelardi T, et al. Toll-like receptor 9 agonist IMO cooperates with cetuximab in K-ras mutant colorectal and pancreatic cancers. Clin Cancer Res. 2011;17(20):6531–41. https://doi.org/10.1158/1078-0432.ccr-10-3376. Northfelt DW, Ramanathan RK, Cohen PA, Von Hoff DD, Weiss GJ, Dietsch GN, et al. A phase I dose-finding study of the novel Toll-like receptor 8 agonist VTX-2337 in adult subjects with advanced solid tumors or lymphoma. Clin Cancer Res. 2014;20(14):3683–91. https://doi.org/10.1158/1078-0432.ccr-14-0392. Migliardi G, Sassi F, Torti D, Galimi F, Zanella ER, Buscarino M, et al. Inhibition of MEK and PI3K/mTOR suppresses tumor growth but does not cause tumor regression in patient-derived xenografts of RAS-mutant colorectal carcinomas. Clin Cancer Res. 2012;18(9):2515–25. https://doi.org/10.1158/1078-0432.ccr-11-2683. Yang H, Higgins B, Kolinsky K, Packman K, Bradley WD, Lee RJ, et al. Antitumor activity of BRAF inhibitor vemurafenib in preclinical models of BRAF-mutant colorectal cancer. Can Res. 2012;72(3):779–89. https://doi.org/10.1158/0008-5472.can-11-2941. Poindessous V, Ouaret D, El Ouadrani K, Battistella A, Mégalophonos VF, Kamsu-Kom N, et al. EGFR- and VEGF(R)-targeted small molecules show synergistic activity in colorectal cancer models refractory to combinations of monoclonal antibodies. Clin Cancer Res. 2011;17(20):6522–30. https://doi.org/10.1158/1078-0432.ccr-11-1607. Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10(6):352–61. https://doi.org/10.1038/nrgastro.2013.35. Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med. 2017;49(5):e338. https://doi.org/10.1038/emm.2017.20. Gallimore AM, Godkin A. Epithelial barriers, microbiota, and colorectal cancer. N Engl J Med. 2013;368(3):282–4. https://doi.org/10.1056/NEJMcibr1212341. Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci USA. 2014;111(51):18321–6. https://doi.org/10.1073/pnas.1406199111. Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015;60(2):208–15. https://doi.org/10.1093/cid/ciu787. Hurtado CG, Wan F, Housseau F, Sears CL. Roles for interleukin 17 and adaptive immunity in pathogenesis of colorectal cancer. Gastroenterology. 2018;155(6):1706–15. https://doi.org/10.1053/j.gastro.2018.08.056. Chung L, Thiele Orberg E, Geis AL, Chan JL, Fu K, DeStefano Shields CE, et al. Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host Microbe. 2018;23(2):203–14.e5. https://doi.org/10.1016/j.chom.2018.01.007. De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34(27):3493–503. https://doi.org/10.1038/onc.2014.286. Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):17023. https://doi.org/10.1038/sigtrans.2017.23. Lee H, Jeong AJ, Ye SK. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019;52(7):415–23. https://doi.org/10.5483/BMBRep.2019.52.7.152. Hubbard JM, Grothey A. Napabucasin: an update on the first-in-class cancer stemness inhibitor. Drugs. 2017;77(10):1091–103. https://doi.org/10.1007/s40265-017-0759-4. Wu MM, Zhang Z, Tong CWS, Yan VW, Cho WCS, To KKW. Repurposing of niclosamide as a STAT3 inhibitor to enhance the anticancer effect of chemotherapeutic drugs in treating colorectal cancer. Life Sci. 2020;262: 118522. https://doi.org/10.1016/j.lfs.2020.118522. Jonker DJ, Nott L, Yoshino T, Gill S, Shapiro J, Ohtsu A, et al. Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(4):263–70. https://doi.org/10.1016/s2468-1253(18)30009-8. Taniguchi H, Masuishi T, Kawazoe A, Muro K, Kadowaki S, Bando H, et al. Phase I study of napabucasin in combination with FOLFIRI + bevacizumab in Japanese patients with metastatic colorectal cancer. Int J Clin Oncol. 2021;26(11):2017–24. https://doi.org/10.1007/s10147-021-01987-9. Li R, Zhou R, Wang H, Li W, Pan M, Yao X, et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019;26(11):2447–63. https://doi.org/10.1038/s41418-019-0312-y. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discovery. 2012;11(10):763–76. https://doi.org/10.1038/nrd3794. Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18(1):64. https://doi.org/10.1186/s12943-019-0976-4. Schimek V, Strasser K, Beer A, Göber S, Walterskirchen N, Brostjan C, et al. Tumour cell apoptosis modulates the colorectal cancer immune microenvironment via interleukin-8-dependent neutrophil recruitment. Cell Death Dis. 2022;13(2):113. https://doi.org/10.1038/s41419-022-04585-3. Bakshi HA, Quinn GA, Nasef MM, Mishra V, Aljabali AAA, El-Tanani M, et al. Crocin inhibits angiogenesis and metastasis in colon cancer via TNF-α/NF-kB/VEGF pathways. Cells. 2022;11(9):1502. https://doi.org/10.3390/cells11091502. Perez LG, Kempski J, McGee HM, Pelzcar P, Agalioti T, Giannou A, et al. TGF-β signaling in Th17 cells promotes IL-22 production and colitis-associated colon cancer. Nat Commun. 2020;11(1):2608. https://doi.org/10.1038/s41467-020-16363-w. Khare V, Paul G, Movadat O, Frick A, Jambrich M, Krnjic A, et al. IL10R2 Overexpression Promotes IL22/STAT3 Signaling in Colorectal Carcinogenesis. Cancer Immunol Res. 2015;3(11):1227–35. https://doi.org/10.1158/2326-6066.Cir-15-0031. Liu Q, Yang C, Wang S, Shi D, Wei C, Song J, et al. Wnt5a-induced M2 polarization of tumor-associated macrophages via IL-10 promotes colorectal cancer progression. Cell Commun Signal. 2020;18(1):51. https://doi.org/10.1186/s12964-020-00557-2. Sullivan KM, Jiang X, Guha P, Lausted C, Carter JA, Hsu C, et al. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut. 2023;72(2):325–37. https://doi.org/10.1136/gutjnl-2021-325808. Cabrero-de Las Heras S, Martínez-Balibrea E. CXC family of chemokines as prognostic or predictive biomarkers and possible drug targets in colorectal cancer. World J Gastroenterol. 2018;24(42):4738–49. https://doi.org/10.3748/wjg.v24.i42.4738. Walz DA, Wu VY, de Lamo R, Dene H, McCoy LE. Primary structure of human platelet factor 4. Thromb Res. 1977;11(6):893–8. https://doi.org/10.1016/0049-3848(77)90117-7. Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61–71. https://doi.org/10.1016/j.cytogfr.2016.08.002. Li E, Yang X, Du Y, Wang G, Chan DW, Wu D, et al. CXCL8 Associated dendritic cell activation marker expression and recruitment as indicators of favorable outcomes in colorectal cancer. Front Immunol. 2021;12:667177. https://doi.org/10.3389/fimmu.2021.667177. Ogawa R, Yamamoto T, Hirai H, Hanada K, Kiyasu Y, Nishikawa G, et al. Loss of SMAD4 promotes colorectal cancer progression by recruiting tumor-associated neutrophils via the CXCL1/8-CXCR2 axis. Clin Cancer Res. 2019;25(9):2887–99. https://doi.org/10.1158/1078-0432.Ccr-18-3684. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–95. https://doi.org/10.1016/j.immuni.2013.10.003. Zhao Q, Guo J, Wang G, Bi Y, Cheng X, Liao Y, et al. CXCL13 promotes intestinal tumorigenesis through the activation of epithelial AKT signaling. Cancer Lett. 2021;511:1–14. https://doi.org/10.1016/j.canlet.2021.04.012. Bandapalli OR, Ehrmann F, Ehemann V, Gaida M, Macher-Goep**er S, Wente M, et al. Down-regulation of CXCL1 inhibits tumor growth in colorectal liver metastasis. Cytokine. 2012;57(1):46–53. https://doi.org/10.1016/j.cyto.2011.10.019. Cui C, Zhang R, Gu F, Pei Y, Sun L, Huang Y, et al. Plasma CXCL3 levels are associated with tumor progression and an unfavorable colorectal cancer prognosis. J Immunol Res. 2022;2022:1336509. https://doi.org/10.1155/2022/1336509. Lin K, Zou R, Lin F, Zheng S, Shen X, Xue X. Expression and effect of CXCL14 in colorectal carcinoma. Mol Med Rep. 2014;10(3):1561–8. https://doi.org/10.3892/mmr.2014.2343. Wang D, Wang X, Si M, Yang J, Sun S, Wu H, et al. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett. 2020;474:36–52. https://doi.org/10.1016/j.canlet.2020.01.005. Braoudaki M, Ahmad MS, Mustafov D, Seriah S, Siddiqui MN, Siddiqui SS. Chemokines and chemokine receptors in colorectal cancer; multifarious roles and clinical impact. Semin Cancer Biol. 2022;86(Pt 2):436–49. https://doi.org/10.1016/j.semcancer.2022.06.002. Kiyasu Y, Kawada K, Hirai H, Ogawa R, Hanada K, Masui H, et al. Disruption of CCR1-mediated myeloid cell accumulation suppresses colorectal cancer progression in mice. Cancer Lett. 2020;487:53–62. https://doi.org/10.1016/j.canlet.2020.05.028. Suarez-Carmona M, Chaorentong P, Kather JN, Rothenheber R, Ahmed A, Berthel A, et al. CCR5 status and metastatic progression in colorectal cancer. Oncoimmunology. 2019;8(9):e1626193. https://doi.org/10.1080/2162402x.2019.1626193. Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell. 2016;29(4):587–601. https://doi.org/10.1016/j.ccell.2016.03.005. Ward ST, Li KK, Hepburn E, Weston CJ, Curbishley SM, Reynolds GM, et al. The effects of CCR5 inhibition on regulatory T-cell recruitment to colorectal cancer. Br J Cancer. 2015;112(2):319–28. https://doi.org/10.1038/bjc.2014.572. Haag GM, Springfeld C, Grün B, Apostolidis L, Zschäbitz S, Dietrich M, et al. Pembrolizumab and maraviroc in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer - The PICCASSO phase I trial. Eur J Cancer (Oxford, England : 1990). 2022;167:112–22. https://doi.org/10.1016/j.ejca.2022.03.017. Raskov H, Orhan A, Gaggar S, Gögenur I. Neutrophils and polymorphonuclear myeloid-derived suppressor cells: an emerging battleground in cancer therapy. Oncogenesis. 2022;11(1):22. https://doi.org/10.1038/s41389-022-00398-3. Fox V, Gokhale PJ, Walsh JR, Matin M, Jones M, Andrews PW. Cell-cell signaling through NOTCH regulates human embryonic stem cell proliferation. Stem cells (Dayton, Ohio). 2008;26(3):715–23. https://doi.org/10.1634/stemcells.2007-0368. Sjölund J, Manetopoulos C, Stockhausen MT, Axelson H. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer (Oxford, England : 1990). 2005;41(17):2620–9. https://doi.org/10.1016/j.ejca.2005.06.025. Dang TP. Notch, apoptosis and cancer. Adv Exp Med Biol. 2012;727:199–209. https://doi.org/10.1007/978-1-4614-0899-4_15. Huang Q, Li J, Zheng J, Wei A. The carcinogenic role of the Notch signaling pathway in the development of hepatocellular carcinoma. J Cancer. 2019;10(6):1570–9. https://doi.org/10.7150/jca.26847. Obata Y, Takahashi D, Ebisawa M, Kakiguchi K, Yonemura S, **nohara T, et al. Epithelial cell-intrinsic Notch signaling plays an essential role in the maintenance of gut immune homeostasis. J Immunol (Baltimore, Md : 1950). 2012;188(5):2427–36. https://doi.org/10.4049/jimmunol.1101128. Gao J, Liu J, Fan D, Xu H, **ong Y, Wang Y, et al. Up-regulated expression of Notch1 and Jagged1 in human colon adenocarcinoma. Parodontol. 2011;59(6):298–302. https://doi.org/10.1016/j.patbio.2010.11.001. Beumer J, Clevers H. Cell fate specification and differentiation in the adult mammalian intestine. Nat Rev Mol Cell Biol. 2021;22(1):39–53. https://doi.org/10.1038/s41580-020-0278-0. Liao W, Li G, You Y, Wan H, Wu Q, Wang C, et al. Antitumor activity of Notch-1 inhibition in human colorectal carcinoma cells. Oncol Rep. 2018;39(3):1063–71. https://doi.org/10.3892/or.2017.6176. Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ, He LY. siRNA-mediated silencing of Notch-1 enhances docetaxel induced mitotic arrest and apoptosis in prostate cancer cells. Asian Pac J Cancer Prev. 2012;13(6):2485–9. https://doi.org/10.7314/apjcp.2012.13.6.2485. Jackstadt R, van Hooff SR, Leach JD, Cortes-Lavaud X, Lohuis JO, Ridgway RA, et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell. 2019;36(3):319–36.e7. https://doi.org/10.1016/j.ccell.2019.08.003. Tyagi A, Sharma AK, Damodaran C. A review on Notch signaling and colorectal cancer. Cells. 2020;9(6):1549. https://doi.org/10.3390/cells9061549. Kukcinaviciute E, Jonusiene V, Sasnauskiene A, Dabkeviciene D, Eidenaite E, Laurinavicius A. Significance of Notch and Wnt signaling for chemoresistance of colorectal cancer cells HCT116. J Cell Biochem. 2018;119(7):5913–20. https://doi.org/10.1002/jcb.26783. Shih Ie M, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Can Res. 2007;67(5):1879–82. https://doi.org/10.1158/0008-5472.Can-06-3958. Krishnaswamy S, Verdile G, Groth D, Kanyenda L, Martins RN. The structure and function of Alzheimer’s gamma secretase enzyme complex. Crit Rev Clin Lab Sci. 2009;46(5–6):282–301. https://doi.org/10.3109/10408360903335821. Gu Y, Masiero M, Banham AH. Notch signaling: its roles and therapeutic potential in hematological malignancies. Oncotarget. 2016;7(20):29804–23. https://doi.org/10.18632/oncotarget.7772. Shao H, Huang Q, Liu ZJ. Targeting Notch signaling for cancer therapeutic intervention. Adv Pharmacol (San Diego, Calif). 2012;65:191–234. https://doi.org/10.1016/b978-0-12-397927-8.00007-5. McCaw TR, Inga E, Chen H, Jaskula-Sztul R, Dudeja V, Bibb JA, et al. Gamma secretase inhibitors in cancer: a current perspective on clinical performance. Oncologist. 2021;26(4):e608–21. https://doi.org/10.1002/onco.13627. Fender AW, Nutter JM, Fitzgerald TL, Bertrand FE, Sigounas G. Notch-1 promotes stemness and epithelial to mesenchymal transition in colorectal cancer. J Cell Biochem. 2015;116(11):2517–27. https://doi.org/10.1002/jcb.25196. Vinson KE, George DC, Fender AW, Bertrand FE, Sigounas G. The Notch pathway in colorectal cancer. Int J Cancer. 2016;138(8):1835–42. https://doi.org/10.1002/ijc.29800. Sonoshita M, Itatani Y, Kakizaki F, Sakimura K, Terashima T, Katsuyama Y, et al. Promotion of colorectal cancer invasion and metastasis through activation of NOTCH-DAB1-ABL-RHOGEF protein TRIO. Cancer Discov. 2015;5(2):198–211. https://doi.org/10.1158/2159-8290.cd-14-0595. Gonulcu SC, Unal B, Bassorgun IC, Ozcan M, Coskun HS, Elpek GO. Expression of Notch pathway components (Numb, Itch, and Siah-1) in colorectal tumors: a clinicopathological study. World J Gastroenterol. 2020;26(26):3814–33. https://doi.org/10.3748/wjg.v26.i26.3814. Qu X, Zhao L, Wang M, Zhang R, Cheng L, Qiu L, et al. Novel functional variants in the Notch pathway and survival of Chinese colorectal cancer. Int J Cancer. 2021;149(1):84–96. https://doi.org/10.1002/ijc.33561. Ye YC, Zhao JL, Lu YT, Gao CC, Yang Y, Liang SQ, et al. NOTCH signaling via WNT regulates the proliferation of alternative, CCR2-independent tumor-associated macrophages in hepatocellular carcinoma. Can Res. 2019;79(16):4160–72. https://doi.org/10.1158/0008-5472.can-18-1691. Wang L, Yu S, Chan ER, Chen KY, Liu C, Che D, et al. Notch-regulated dendritic cells restrain inflammation-associated colorectal carcinogenesis. Cancer Immunol Res. 2021;9(3):348–61. https://doi.org/10.1158/2326-6066.cir-20-0428. Yu W, Wang Y, Guo P. Notch signaling pathway dampens tumor-infiltrating CD8 (+) T cells activity in patients with colorectal carcinoma. Biomed Pharmacother Biomed Pharmacother. 2018;97:535–42. https://doi.org/10.1016/j.biopha.2017.10.143. Wang F, Long J, Li L, Zhao ZB, Wei F, Yao Y, et al. Mutations in the notch signalling pathway are associated with enhanced anti-tumour immunity in colorectal cancer. J Cell Mol Med. 2020;24(20):12176–87. https://doi.org/10.1111/jcmm.15867. Long J, Wang D, Yang X, Wang A, Lin Y, Zheng M, et al. Identification of NOTCH4 mutation as a response biomarker for immune checkpoint inhibitor therapy. BMC Med. 2021;19(1):154. https://doi.org/10.1186/s12916-021-02031-3. Zhang H, Jiang H, Chen L, Liu J, Hu X, Zhang H. Inhibition of Notch1/Hes1 signaling pathway improves radiosensitivity of colorectal cancer cells. Eur J Pharmacol. 2018;818:364–70. https://doi.org/10.1016/j.ejphar.2017.11.009. Aleksic T, Feller SM. Gamma-secretase inhibition combined with platinum compounds enhances cell death in a large subset of colorectal cancer cells. Cell Commun Signal. 2008;6:8. https://doi.org/10.1186/1478-811x-6-8. Hanna GJ, Stathis A, Lopez-Miranda E, Racca F, Quon D, Leyvraz S, et al. A phase I study of the Pan-Notch inhibitor CB-103 for patients with advanced Adenoid cystic carcinoma and other tumors. Cancer Res Commun. 2023;3(9):1853–61. https://doi.org/10.1158/2767-9764.Crc-23-0333. Astudillo L, Da Silva TG, Wang Z, Han X, ** K, VanWye J, et al. The small molecule IMR-1 inhibits the Notch transcriptional activation complex to suppress tumorigenesis. Can Res. 2016;76(12):3593–603. https://doi.org/10.1158/0008-5472.Can-16-0061. Takahashi-Yanaga F, Sasaguri T. The Wnt/beta-catenin signaling pathway as a target in drug discovery. J Pharmacol Sci. 2007;104(4):293–302. https://doi.org/10.1254/jphs.cr0070024. Nie X, Liu H, Liu L, Wang YD, Chen WD. Emerging roles of Wnt Ligands in human colorectal cancer. Front Oncol. 2020;10:1341. https://doi.org/10.3389/fonc.2020.01341. Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25(57):7461–8. https://doi.org/10.1038/sj.onc.1210053. Koni M, Pinnarò V, Brizzi MF. The Wnt signalling pathway: a tailored target in cancer. Int J Mol Sci. 2020;21(20):7697. https://doi.org/10.3390/ijms21207697. Leung RWH, Lee TKW. Wnt/β-Catenin signaling as a driver of Stemness and metabolic reprogramming in hepatocellular carcinoma. Cancers. 2022;14(21):5468. https://doi.org/10.3390/cancers14215468. Liu J, **ao Q, **ao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. https://doi.org/10.1038/s41392-021-00762-6. Bian J, Dannappel M, Wan C, Firestein R. Transcriptional regulation of Wnt/β-Catenin pathway in colorectal cancer. Cells. 2020;9(9):2125. https://doi.org/10.3390/cells9092125. Sebio A, Kahn M, Lenz HJ. The potential of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther Targets. 2014;18(6):611–5. https://doi.org/10.1517/14728222.2014.906580. Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular mechanisms of colon cancer progression and metastasis: recent insights and advancements. Int J Mol Sci. 2020;22(1):130. https://doi.org/10.3390/ijms22010130. Farooqi AA, de la Roche M, Djamgoz MBA, Siddik ZH. Overview of the oncogenic signaling pathways in colorectal cancer: Mechanistic insights. Semin Cancer Biol. 2019;58:65–79. https://doi.org/10.1016/j.semcancer.2019.01.001. Cheng X, Xu X, Chen D, Zhao F, Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother = Biomed Pharmacother. 2019;110:473–81. https://doi.org/10.1016/j.biopha.2018.11.082. Ren Q, Chen J, Liu Y. LRP5 and LRP6 in Wnt signaling: similarity and divergence. Front Cell Dev Biol. 2021;9:670960. https://doi.org/10.3389/fcell.2021.670960. Hayat R, Manzoor M, Hussain A. Wnt signaling pathway: a comprehensive review. Cell Biol Int. 2022;46(6):863–77. https://doi.org/10.1002/cbin.11797. Jung YS, Park JI. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med. 2020;52(2):183–91. https://doi.org/10.1038/s12276-020-0380-6. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. https://doi.org/10.1016/j.devcel.2009.06.016. Konrad CV, Murali R, Varghese BA, Nair R. The role of cancer stem cells in tumor heterogeneity and resistance to therapy. Can J Physiol Pharmacol. 2017;95(1):1–15. https://doi.org/10.1139/cjpp-2016-0079. Oliveira LFS, Predes D, Borges HL, Abreu JG. Therapeutic potential of naturally occurring small molecules to target the Wnt/β-Catenin signaling pathway in colorectal cancer. Cancers. 2022;14(2):403. https://doi.org/10.3390/cancers14020403. Prokhnevska N, Cardenas MA, Valanparambil RM, Sobierajska E, Barwick BG, Jansen C, et al. CD8(+) T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity. 2023;56(1):107–24.e5. https://doi.org/10.1016/j.immuni.2022.12.002. Connolly KA, Kuchroo M, Venkat A, Khatun A, Wang J, William I, et al. A reservoir of stem-like CD8(+) T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci Immunol. 2021;6(64):7836. https://doi.org/10.1126/sciimmunol.abg7836. Zhou Y, Xu J, Luo H, Meng X, Chen M, Zhu D. Wnt signaling pathway in cancer immunotherapy. Cancer Lett. 2022;525:84–96. https://doi.org/10.1016/j.canlet.2021.10.034. Wang B, Tian T, Kalland KH, Ke X, Qu Y. Targeting Wnt/β-Catenin signaling for cancer immunotherapy. Trends Pharmacol Sci. 2018;39(7):648–58. https://doi.org/10.1016/j.tips.2018.03.008. van Loosdregt J, Coffer PJ. The role of WNT signaling in mature T cells: T cell factor is coming home. J Immunol (Baltimore, Md : 1950). 2018;201(8):2193–200. https://doi.org/10.4049/jimmunol.1800633. Wong C, Chen C, Wu Q, Liu Y, Zheng P. A critical role for the regulated wnt-myc pathway in naive T cell survival. J Immunol (Baltimore, Md : 1950). 2015;194(1):158–67. https://doi.org/10.4049/jimmunol.1401238. van Loosdregt J, Fleskens V, Tiemessen MM, Mokry M, van Boxtel R, Meerding J, et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity. 2013;39(2):298–310. https://doi.org/10.1016/j.immuni.2013.07.019. Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, et al. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. 2021;6(1):307. https://doi.org/10.1038/s41392-021-00701-5. Shah K, Panchal S, Patel B. Porcupine inhibitors: Novel and emerging anti-cancer therapeutics targeting the Wnt signaling pathway. Pharmacol Res. 2021;167:105532. https://doi.org/10.1016/j.phrs.2021.105532. Davis SL, Cardin DB, Shahda S, Lenz HJ, Dotan E, O’Neil BH, et al. A phase 1b dose escalation study of Wnt pathway inhibitor vantictumab in combination with nab-paclitaxel and gemcitabine in patients with previously untreated metastatic pancreatic cancer. Invest New Drugs. 2020;38(3):821–30. https://doi.org/10.1007/s10637-019-00824-1. Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J, et al. A first-in-human phase I study of the anticancer stem cell agent Ipafricept (OMP-54F28), a decoy receptor for Wnt Ligands, in patients with advanced solid tumors. Clin Cancer Res. 2017;23(24):7490–7. https://doi.org/10.1158/1078-0432.Ccr-17-2157. Moore KN, Gunderson CC, Sabbatini P, McMeekin DS, Mantia-Smaldone G, Burger RA, et al. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 2019;154(2):294–301. https://doi.org/10.1016/j.ygyno.2019.04.001. DeVito NC, Sturdivant M, Thievanthiran B, **ao C, Plebanek MP, Salama AKS, et al. Pharmacological Wnt ligand inhibition overcomes key tumor-mediated resistance pathways to anti-PD-1 immunotherapy. Cell Rep. 2021;35(5):109071. https://doi.org/10.1016/j.celrep.2021.109071. Rodon J, Argilés G, Connolly RM, Vaishampayan U, de Jonge M, Garralda E, et al. Phase 1 study of single-agent WNT974, a first-in-class Porcupine inhibitor, in patients with advanced solid tumours. Br J Cancer. 2021;125(1):28–37. https://doi.org/10.1038/s41416-021-01389-8. Wang C, Dai J, Sun Z, Shi C, Cao H, Chen X, et al. Targeted inhibition of disheveled PDZ domain via NSC668036 depresses fibrotic process. Exp Cell Res. 2015;331(1):115–22. https://doi.org/10.1016/j.yexcr.2014.10.023. Zhang K, Li M, Huang H, Li L, Yang J, Feng L, et al. Dishevelled1–3 contribute to multidrug resistance in colorectal cancer via activating Wnt/β-catenin signaling. Oncotarget. 2017;8(70):115803–16. https://doi.org/10.18632/oncotarget.23253. Tufail M, Wu C. Wnt3a is a promising target in colorectal cancer. Med Oncol (Northwood, London, England). 2023;40(3):86. https://doi.org/10.1007/s12032-023-01958-2. Yan R, Zhu H, Huang P, Yang M, Shen M, Pan Y, et al. Liquidambaric acid inhibits Wnt/β-catenin signaling and colon cancer via targeting TNF receptor-associated factor 2. Cell Rep. 2022;38(5):110319. https://doi.org/10.1016/j.celrep.2022.110319. Qu Y, Olsen JR, Yuan X, Cheng PF, Levesque MP, Brokstad KA, et al. Small molecule promotes β-catenin citrullination and inhibits Wnt signaling in cancer. Nat Chem Biol. 2018;14(1):94–101. https://doi.org/10.1038/nchembio.2510. Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Can Res. 2012;72(11):2822–32. https://doi.org/10.1158/0008-5472.Can-11-3336. Yu J, Liu D, Sun X, Yang K, Yao J, Cheng C, et al. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019;10(1):26. https://doi.org/10.1038/s41419-018-1263-9. Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, et al. Multicenter phase I/II trial of Napabucasin and Pembrolizumab in patients with metastatic colorectal cancer (EPOC1503/SCOOP Trial). Clin Cancer Res. 2020;26(22):5887–94. https://doi.org/10.1158/1078-0432.Ccr-20-1803. Feng M, Zhao Z, Yang M, Ji J, Zhu D. T-cell-based immunotherapy in colorectal cancer. Cancer Lett. 2021;498:201–9. https://doi.org/10.1016/j.canlet.2020.10.040. Wang J, **ang H, Lu Y, Wu T. Role and clinical significance of TGF-β1 and TGF-βR1 in malignant tumors (Review). Int J Mol Med. 2021;47(4):55. https://doi.org/10.3892/ijmm.2021.4888. Liu Q, Zhao RM, Wang DY, Li P, Qu YF, Ji X. Genome-wide characterization of the TGF-β gene family and their expression in different tissues during tail regeneration in the Schlegel’s Japanese gecko Gekko japonicus. Int J Biol Macromol. 2023;255:128127. https://doi.org/10.1016/j.ijbiomac.2023.128127. Massagué J, Sheppard D. TGF-β signaling in health and disease. Cell. 2023;186(19):4007–37. https://doi.org/10.1016/j.cell.2023.07.036. Wang Q, **ong F, Wu G, Wang D, Liu W, Chen J, et al. SMAD proteins in TGF-β signalling pathway in cancer: regulatory mechanisms and clinical applications. Diagnostics (Basel, Switzerland). 2023;13(17):2769. https://doi.org/10.3390/diagnostics13172769. Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. https://doi.org/10.1016/s0092-8674(00)00121-5. Mukherjee P, Winter SL, Alexandrow MG. Cell cycle arrest by transforming growth factor beta1 near G1/S is mediated by acute abrogation of prereplication complex activation involving an Rb-MCM interaction. Mol Cell Biol. 2010;30(3):845–56. https://doi.org/10.1128/mcb.01152-09. Zhang Z, Zhu X. MiR-103a-3p contributes to the progression of colorectal cancer by regulating GREM2 expression. Yonsei Med J. 2022;63(6):520–9. https://doi.org/10.3349/ymj.2022.63.6.520. Zhao J, Li G, Wei J, Dang S, Yu X, Ding L, et al. Ellagic acid induces cell cycle arrest and apoptosis via the TGF-β1/Smad3 signaling pathway in human colon cancer HCT-116 cells. Oncol Rep. 2020;44(2):768–76. https://doi.org/10.3892/or.2020.7617. Seoane J, Gomis RR. TGF-β family signaling in tumor suppression and cancer progression. Cold Spring Harbor Perspect Biol. 2017;9(12):e022277. https://doi.org/10.1101/cshperspect.a022277. Villalba M, Evans SR, Vidal-Vanaclocha F, Calvo A. Role of TGF-β in metastatic colon cancer: it is finally time for targeted therapy. Cell Tissue Res. 2017;370(1):29–39. https://doi.org/10.1007/s00441-017-2633-9. Tamayo E, Alvarez P, Merino R. TGFβ superfamily members as regulators of B cell development and function-implications for autoimmunity. Int J Mol Sci. 2018;19(12):3928. https://doi.org/10.3390/ijms19123928. Khalili-Tanha G, Fiuji H, Gharib M, Moghbeli M, Khalili-Tanha N, Rahmani F, et al. Dual targeting of TGF-β and PD-L1 inhibits tumor growth in TGF-β/PD-L1-driven colorectal carcinoma. Life Sci. 2023;328:121865. https://doi.org/10.1016/j.lfs.2023.121865. Jamialahmadi H, Nazari SE, TanzadehPanah H, Saburi E, Asgharzadeh F, Khojasteh-Leylakoohi F, et al. Targeting transforming growth factor beta (TGF-β) using Pirfenidone, a potential repurposing therapeutic strategy in colorectal cancer. Sci Rep. 2023;13(1):14357. https://doi.org/10.1038/s41598-023-41550-2. Li X, Wu Y, Tian T. TGF-β Signaling in Metastatic Colorectal Cancer (mCRC): from underlying mechanism to potential applications in clinical development. Int J Mol Sci. 2022;23(22):14436. https://doi.org/10.3390/ijms232214436. Zhu M, Jiang B, Yan D, Wang X, Ge H, Sun Y. Knockdown of TMEM45A overcomes multidrug resistance and epithelial-mesenchymal transition in human colorectal cancer cells through inhibition of TGF-β signalling pathway. Clin Exp Pharmacol Physiol. 2020;47(3):503–16. https://doi.org/10.1111/1440-1681.13220. Martin CJ, Datta A, Littlefield C, Kalra A, Chapron C, Wawersik S, et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci Transl Med. 2020;12(536):eaay8456. https://doi.org/10.1126/scitranslmed.aay8456. Ciardiello D, Blauensteiner B, Matrone N, Belli V, Mohr T, Vitiello PP, et al. Dual inhibition of TGFβ and AXL as a novel therapy for human colorectal adenocarcinoma with mesenchymal phenotype. Med Oncol (Northwood, London, England). 2021;38(3):24. https://doi.org/10.1007/s12032-021-01464-3. Wang H, Chen M, Sang X, You X, Wang Y, Paterson IC, et al. Development of small molecule inhibitors targeting TGF-β ligand and receptor: Structures, mechanism, preclinical studies and clinical usage. Eur J Med Chem. 2020;191:112154. https://doi.org/10.1016/j.ejmech.2020.112154. Zhang BH, Wang C, Dong W, Chen X, Leng C, Luo X, et al. A novel approach for monitoring TGF-β signaling in vivo in colon cancer. Carcinogenesis. 2021;42(4):631–9. https://doi.org/10.1093/carcin/bgaa142. Kaur G, Kyte D, Reeve BB, Basch E, Calvert M. Patient-reported outcome monitoring in a routine paediatric oncology setting: challenges and opportunities. Lancet Oncol. 2019;20(1):19–20. https://doi.org/10.1016/s1470-2045(18)30785-x. Huyghe N, Benidovskaya E, Stevens P, Van den Eynde M. Biomarkers of response and resistance to immunotherapy in microsatellite stable colorectal cancer: toward a new personalized medicine. Cancers. 2022;14(9):2241. https://doi.org/10.3390/cancers14092241. Binabaj MM, Asgharzadeh F, Rahmani F, Al-Asady AM, Hashemzehi M, Soleimani A, et al. Vactosertib potently improves anti-tumor properties of 5-FU for colon cancer. Daru. 2023;31(2):193–203. https://doi.org/10.1007/s40199-023-00474-y. Lee KW, Park YS, Ahn JB, et al. 332 Novel TGF-β signatures in metastatic colorectal cancer patients treated with vactosertib in combination with pembrolizumab. J ImmunoTher Cancer. 2020. https://doi.org/10.1136/JITC-2020-SITC2020.0332. Kim TW, Lee K-W, Ahn JB, Park YS, Chan-Young O, Park H, et al. 74 Tumor microenvironment based on PD-L1 and CD8 T-cell infiltration correlated with the response of MSS mCRC patients treated vactosertib in combination with pembrolizumab. J ImmunoTher Cancer. 2021;9(Suppl 2):A82–A. Selfridge JE, Bajor DL, Mohamed A, Chakrabarti S, Reese J, Lazarus HM, et al. Trial in progress: Natural killer (NK) cells with TGFβ receptor I inhibitor vactosertib and IL-2 in patients with metastatic colorectal cancer or hematologic malignancies. Am Soc Clin Oncol. 2023. https://clinicaltrials.gov/study/NCT05400122. Cabibbo G, Celsa C, Enea M, Battaglia S, Rizzo GEM, Grimaudo S, et al. Optimizing sequential systemic therapies for advanced hepatocellular carcinoma: a decision analysis. Cancers. 2020;12(8):2132. https://doi.org/10.3390/cancers12082132. Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284(15):1954–61. https://doi.org/10.1001/jama.284.15.1954. Mittmann N, Au HJ, Tu D, O’Callaghan CJ, Isogai PK, Karapetis CS, et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: evaluation of National Cancer Institute of Canada Clinical Trials Group CO.17 trial. J Natl Cancer Inst. 2009;101(17):1182–92. https://doi.org/10.1093/jnci/djp232. Behl AS, Goddard KA, Flottemesch TJ, Veenstra D, Meenan RT, Lin JS, et al. Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer. J Natl Cancer Inst. 2012;104(23):1785–95. https://doi.org/10.1093/jnci/djs433. Yabroff KR, Schrag D. Challenges and opportunities for use of cost-effectiveness analysis. J Natl Cancer Inst. 2009;101(17):1161–3. https://doi.org/10.1093/jnci/djp258. Dolgin E. FDA narrows drug label usage. Nature. 2009;460(7259):1069. https://doi.org/10.1038/4601069a. Blank PR, Moch H, Szucs TD, Schwenkglenks M. KRAS and BRAF mutation analysis in metastatic colorectal cancer: a cost-effectiveness analysis from a Swiss perspective. Clin Cancer Res. 2011;17(19):6338–46. https://doi.org/10.1158/1078-0432.ccr-10-2267. Tsuchihashi Z, Khambata-Ford S, Hanna N, Jänne PA. Responsiveness to cetuximab without mutations in EGFR. N Engl J Med. 2005;353(2):208–9. https://doi.org/10.1056/nejm200507143530218. Lawrence D, Maschio M, Leahy KJ, Yunger S, Easaw JC, Weinstein MC. Economic analysis of bevacizumab, cetuximab, and panitumumab with fluoropyrimidine-based chemotherapy in the first-line treatment of KRAS wild-type metastatic colorectal cancer (mCRC). J Med Econ. 2013;16(12):1387–98. https://doi.org/10.3111/13696998.2013.852097. Zhang PF, Wen F, Zhou J, Huang JX, Zhou KX, Wu QJ, et al. Cost-effectiveness analysis of capecitabine plus bevacizumab versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer from Chinese societal perspective. Clin Transl Oncol. 2020;22(1):103–10. https://doi.org/10.1007/s12094-019-02114-x. Sherman SK, Lange JJ, Dahdaleh FS, Rajeev R, Gamblin TC, Polite BN, et al. Cost-effectiveness of maintenance Capecitabine and Bevacizumab for metastatic colorectal cancer. JAMA Oncol. 2019;5(2):236–42. https://doi.org/10.1001/jamaoncol.2018.5070. François E, Mineur L, Deplanque G, Laplaige P, Smith D, Gourgou S, et al. Efficacy and safety of Bevacizumab combined with first-line chemotherapy in elderly (≥75 Years) patients with metastatic colorectal cancer: a real-world study. Clin Colorectal Cancer. 2020;19(3):e100–9. https://doi.org/10.1016/j.clcc.2020.02.009. Goldberg RM, O’Neil BH. Colorectal cancer: Irinotecan therapy-following a trail of breadcrumbs? Nat Rev Gastroenterol Hepatol. 2009;6(9):507–9. https://doi.org/10.1038/nrgastro.2009.140. Foley KA, Wang PF, Barber BL, Long SR, Bagalman JE, Wagner V, et al. Clinical and economic impact of infusion reactions in patients with colorectal cancer treated with cetuximab. Ann Oncol. 2010;21(7):1455–61. https://doi.org/10.1093/annonc/mdp535. Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19(11):671–87. https://doi.org/10.1038/s41576-018-0051-9. Crespo M, Vilar E, Tsai SY, Chang K, Amin S, Srinivasan T, et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med. 2017;23(7):878–84. https://doi.org/10.1038/nm.4355. Che YH, Choi IY, Song CE, Park C, Lim SK, Kim JH, et al. Peripheral neuron-organoid interaction induces colonic epithelial differentiation via non-synaptic substance P secretion. Int J Stem Cells. 2023;16(3):269–80. https://doi.org/10.15283/ijsc23026. Goto T, Iwai H, Kuramoto E, Yamanaka A. Neuropeptides and ATP signaling in the trigeminal ganglion. Jap Dental Sci Rev. 2017;53(4):117–24. https://doi.org/10.1016/j.jdsr.2017.01.003. Lu XF, Li YY, Wang CG, Wei JQ, Ye Y, Zhang LC, et al. Substance P in the cerebrospinal fluid-contacting nucleus contributes to morphine physical dependence in rats. Neurosci Lett. 2011;488(2):188–92. https://doi.org/10.1016/j.neulet.2010.11.026. Toshimitsu K, Takano A, Fujii M, Togasaki K, Matano M, Takahashi S, et al. Organoid screening reveals epigenetic vulnerabilities in human colorectal cancer. Nat Chem Biol. 2022;18(6):605–14. https://doi.org/10.1038/s41589-022-00984-x. Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920–6. https://doi.org/10.1126/science.aao2774. Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, et al. A Colorectal tumor organoid library demonstrates progressive loss of Niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18(6):827–38. https://doi.org/10.1016/j.stem.2016.04.003. Verissimo CS, Overmeer RM, Ponsioen B, Drost J, Mertens S, Verlaan-Klink I, et al. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. Elife. 2016;5:e18489. https://doi.org/10.7554/eLife.18489. **naris C, Brizi V, Remuzzi G. Organoid models and applications in biomedical research. Nephron. 2015;130(3):191–9. https://doi.org/10.1159/000433566. Benelli R, Costa D, Salvini L, Tardito S, Tosetti F, Villa F, et al. Targeting of colorectal cancer organoids with zoledronic acid conjugated to the anti-EGFR antibody cetuximab. J Immunother Cancer. 2022;10(12):e005660. https://doi.org/10.1136/jitc-2022-005660. Mao Y, Wang W, Yang J, Zhou X, Lu Y, Gao J, et al. Drug repurposing screening and mechanism analysis based on human colorectal cancer organoids. Protein Cell. 2023. https://doi.org/10.1093/procel/pwad038. Schütte M, Risch T, Abdavi-Azar N, Boehnke K, Schumacher D, Keil M, et al. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat Commun. 2017;8:14262. https://doi.org/10.1038/ncomms14262. Usui T, Sakurai M, Umata K, Elbadawy M, Ohama T, Yamawaki H, et al. Hedgehog signals mediate anti-cancer drug resistance in three-dimensional primary colorectal cancer organoid culture. Int J Mol Sci. 2018;19(4):1098. https://doi.org/10.3390/ijms19041098. **ong W, Chen Y, Zhang C, Li J, Huang H, Zhu Y, et al. Pharmacologic inhibition of IL11/STAT3 signaling increases MHC-I expression and T cell infiltration. J Transl Med. 2023;21(1):416. https://doi.org/10.1186/s12967-023-04079-6. Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, et al. Personalized In vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017;7(5):462–77. https://doi.org/10.1158/2159-8290.Cd-16-1154. Nikonova AS, Deneka AY, Silva FN, Pirestani S, Tricarico R, Kiseleva AA, et al. Loss of Pkd1 limits susceptibility to colitis and colorectal cancer. Oncogenesis. 2023;12(1):40. https://doi.org/10.1038/s41389-023-00486-y. Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 2020;26(1):17–26.e6. https://doi.org/10.1016/j.stem.2019.10.010. Pasch CA, Favreau PF, Yueh AE, Babiarz CP, Gillette AA, Sharick JT, et al. Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation. Clin Cancer Res. 2019;25(17):5376–87. https://doi.org/10.1158/1078-0432.Ccr-18-3590. Álvarez-Varela A, Novellasdemunt L, Barriga FM, Hernando-Momblona X, Cañellas-Socias A, Cano-Crespo S, et al. Mex3a marks drug-tolerant persister colorectal cancer cells that mediate relapse after chemotherapy. Nature Cancer. 2022;3(9):1052–70. https://doi.org/10.1038/s43018-022-00402-0. Ringel T, Frey N, Ringnalda F, Janjuha S, Cherkaoui S, Butz S, et al. Genome-scale CRISPR screening in human intestinal organoids identifies drivers of TGF-β resistance. Cell Stem Cell. 2020;26(3):431–40.e8. https://doi.org/10.1016/j.stem.2020.02.007. Michels BE, Mosa MH, Streibl BI, Zhan T, Menche C, Abou-El-Ardat K, et al. Pooled in vitro and in vivo CRISPR-Cas9 screening identifies tumor suppressors in human colon organoids. Cell Stem Cell. 2020;26(5):782–92.e7. https://doi.org/10.1016/j.stem.2020.04.003. Schnalzger TE, de Groot MH, Zhang C, Mosa MH, Michels BE, Röder J, et al. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019;38(12):e100928. https://doi.org/10.15252/embj.2018100928. Cattaneo CM, Dijkstra KK, Fanchi LF, Kelderman S, Kaing S, van Rooij N, et al. Tumor organoid-T-cell coculture systems. Nat Protoc. 2020;15(1):15–39. https://doi.org/10.1038/s41596-019-0232-9. Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174(6):1586–98.e12. https://doi.org/10.1016/j.cell.2018.07.009. Gardner CL, Pavel-Dinu M, Dobbs K, Bosticardo M, Reardon PK, Lack J, et al. Gene editing rescues in vitro T cell development of RAG2-deficient induced pluripotent stem cells in an artificial thymic organoid system. J Clin Immunol. 2021;41(5):852–62. https://doi.org/10.1007/s10875-021-00989-6. Parikh AY, Masi R, Gasmi B, Hanada KI, Parkhurst M, Gartner J, et al. Using patient-derived tumor organoids from common epithelial cancers to analyze personalized T-cell responses to neoantigens. Cancer Immunol, Immunother. 2023;72(10):3149–62. https://doi.org/10.1007/s00262-023-03476-6. Fang H, Huang Y, Luo Y, Tang J, Yu M, Zhang Y, et al. SIRT1 induces the accumulation of TAMs at colorectal cancer tumor sites via the CXCR4/CXCL12 axis. Cell Immunol. 2022;371:104458. https://doi.org/10.1016/j.cellimm.2021.104458. Küçükköse E, Heesters BA, Villaudy J, Verheem A, Cercel M, van Hal S, et al. Modeling resistance of colorectal peritoneal metastases to immune checkpoint blockade in humanized mice. J Immunother Cancer. 2022;10(12):e005345. https://doi.org/10.1136/jitc-2022-005345. Pickles OJ, Wanigasooriya K, Ptasinska A, Patel AJ, Robbins HL, Bryer C, et al. MHC class II is induced by IFNγ and follows three distinct patterns of expression in colorectal cancer organoids. Cancer Res Commun. 2023;3(8):1501–13. https://doi.org/10.1158/2767-9764.Crc-23-0091. Strating E, Verhagen MP, Wensink E, Dünnebach E, Wijler L, Aranguren I, et al. Co-cultures of colon cancer cells and cancer-associated fibroblasts recapitulate the aggressive features of mesenchymal-like colon cancer. Front Immunol. 2023;14:1053920. https://doi.org/10.3389/fimmu.2023.1053920. Loree JM, Dowers A, Tu D, Jonker DJ, Edelstein DL, Quinn H, et al. Expanded low allele frequency RAS and BRAF V600E testing in metastatic colorectal cancer as predictive biomarkers for Cetuximab in the randomized CO17 trial. Clin Cancer Res. 2021;27(1):52–9. https://doi.org/10.1158/1078-0432.ccr-20-2710. Chau I, Cunningham D, Hickish T, Massey A, Higgins L, Osborne R, et al. Gefitinib and irinotecan in patients with fluoropyrimidine-refractory, irinotecan-naive advanced colorectal cancer: a phase I-II study. Ann Oncol. 2007;18(4):730–7. https://doi.org/10.1093/annonc/mdl481. Jimeno A, Grávalos C, Escudero P, Sevilla I, Vega-Villegas ME, Alonso V, et al. Phase I/II study of gefitinib and capecitabine in patients with colorectal cancer. Clin Transl Oncol. 2008;10(1):52–7. https://doi.org/10.1007/s12094-008-0153-5. Kozuch P, Malamud S, Wasserman C, Homel P, Mirzoyev T, Grossbard M. Phase II trial of erlotinib and capecitabine for patients with previously untreated metastatic colorectal cancer. Clin Colorectal Cancer. 2009;8(1):38–42. https://doi.org/10.3816/CCC.2009.n.006. Van Cutsem E, Verslype C, Beale P, Clarke S, Bugat R, Rakhit A, et al. A phase Ib dose-escalation study of erlotinib, capecitabine and oxaliplatin in metastatic colorectal cancer patients. Ann Oncol. 2008;19(2):332–9. https://doi.org/10.1093/annonc/mdm452. Vincent MD, Breadner D, Soulieres D, Kerr IG, Sanatani M, Kocha W, et al. Phase II trial of capecitabine plus erlotinib versus capecitabine alone in patients with advanced colorectal cancer. Future Oncol (London, England). 2017;13(9):777–86. https://doi.org/10.2217/fon-2016-0444. Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26(21):3523–9. https://doi.org/10.1200/jco.2007.15.4138. Avallone A, Piccirillo MC, Nasti G, Rosati G, Carlomagno C, Di Gennaro E, et al. Effect of Bevacizumab in combination with standard Oxaliplatin-based regimens in patients with metastatic colorectal cancer: a randomized clinical trial. JAMA Netw Open. 2021;4(7):e2118475. https://doi.org/10.1001/jamanetworkopen.2021.18475. Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26(11):1830–5. https://doi.org/10.1200/jco.2007.13.7679. Overman MJ, Ferrarotto R, Raghav K, George B, Qiao W, Machado KK, et al. The addition of Bevacizumab to Oxaliplatin-based chemotherapy: impact upon Hepatic Sinusoidal injury and Thrombocytopenia. J Natl Cancer Inst. 2018;110(8):888–94. https://doi.org/10.1093/jnci/djx288. Kuijk LM, Verstege MI, Rekers NV, Bruijns SC, Hooijberg E, Roep BO, et al. Notch controls generation and function of human effector CD8+ T cells. Blood. 2013;121(14):2638–46. https://doi.org/10.1182/blood-2012-07-442962. Backer RA, Helbig C, Gentek R, Kent A, Laidlaw BJ, Dominguez CX, et al. A central role for Notch in effector CD8(+) T cell differentiation. Nat Immunol. 2014;15(12):1143–51. https://doi.org/10.1038/ni.3027. Sauma D, Ramirez A, Alvarez K, Rosemblatt M, Bono MR. Notch signalling regulates cytokine production by CD8+ and CD4+ T cells. Scand J Immunol. 2012;75(4):389–400. https://doi.org/10.1111/j.1365-3083.2012.02673.x. Mathieu M, Cotta-Grand N, Daudelin JF, Thébault P, Labrecque N. Notch signaling regulates PD-1 expression during CD8(+) T-cell activation. Immunol Cell Biol. 2013;91(1):82–8. https://doi.org/10.1038/icb.2012.53. Sierra RA, Thevenot P, Raber PL, Cui Y, Parsons C, Ochoa AC, et al. Rescue of notch-1 signaling in antigen-specific CD8+ T cells overcomes tumor-induced T-cell suppression and enhances immunotherapy in cancer. Cancer Immunol Res. 2014;2(8):800–11. https://doi.org/10.1158/2326-6066.cir-14-0021. Verma V, Jafarzadeh N, Boi S, Kundu S, Jiang Z, Fan Y, et al. MEK inhibition reprograms CD8(+) T lymphocytes into memory stem cells with potent antitumor effects. Nat Immunol. 2021;22(1):53–66. https://doi.org/10.1038/s41590-020-00818-9. Zhan T, Ambrosi G, Wandmacher AM, Rauscher B, Betge J, Rindtorff N, et al. MEK inhibitors activate Wnt signalling and induce stem cell plasticity in colorectal cancer. Nat Commun. 2019;10(1):2197. https://doi.org/10.1038/s41467-019-09898-0. LeSavage BL, Suhar RA, Broguiere N, Lutolf MP, Heilshorn SC. Next-generation cancer organoids. Nat Mater. 2022;21(2):143–59. https://doi.org/10.1038/s41563-021-01057-5. Eto T, Miyake K, Nosho K, Ohmuraya M, Imamura Y, Arima K, et al. Impact of loss-of-function mutations at the RNF43 locus on colorectal cancer development and progression. J Pathol. 2018;245(4):445–55. https://doi.org/10.1002/path.5098. Tokumaru Y, Oshi M, Patel A, Tian W, Yan L, Matsuhashi N, et al. Organoids are limited in modeling the colon adenoma-carcinoma sequence. Cells. 2021;10(3):488. https://doi.org/10.3390/cells10030488. Not applicable. Y.S. & M.C. wrote the manuscript. Y.W. drew the figures and tables. X.M. & H.S. provided the conceptual idea and revised the manuscript. All authors have read and approved the final manuscript. Not applicable. The authors declare no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Song, Y., Chen, M., Wei, Y. et al. Signaling pathways in colorectal cancer implications for the target therapies.
Mol Biomed 5, 21 (2024). https://doi.org/10.1186/s43556-024-00178-y Received: Accepted: Published: DOI: https://doi.org/10.1186/s43556-024-00178-yAvailability of data and materials
References
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Competing interests
Additional information
Publisher’s Note
Rights and permissions
About this article
Cite this article
Keywords