Abstract
There is a vast number of treatments on the market for the management of wounds and burns, representing a multi-billion dollar industry worldwide. These include conventional wound dressings, dressings that incorporate growth factors to stimulate and facilitate the wound healing process, and skin substitutes that incorporate patient-derived cells. This article will review the more established, and the recent advances in the use of biomaterials for wound healing therapies, and their future direction.
Similar content being viewed by others
Background
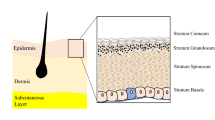
Skin plays a key role in protecting our internal environment from the external environment, maintaining homeostasis, and regulating temperature. On the outer side is the epidermis that consists predominantly of keratinocytes, which form a tight seal for protection (Fig. 1), along with melanocytes, Langerhan and Merkel cells [1]. Below this is the dermis, which is attached to the epidermis by the basement membrane, a thin layer of extracellular matrix (ECM) consisting mostly of laminins, integrins, perlecan, nidogen, and collagen IV [2, 3]. The composition of the dermis is complex and differs quite dramatically from the epidermis [1]. It consists of ECM, which acts as a scaffold for fibroblasts and other mesenchymal cells, blood vessels, hair follicles, and sweat glands [3,4,5]. It also houses molecules, such as growth factors and enzymes, that regulate the local environment [2, 3]. The dermis has several sublayers, with the papillary layer closest to the basement membrane consisting of poorly ordered thin collagen fibers housing a high density of fibroblasts [1]. Sandwiched between the lower dermal white adipose tissue and the papillary layer is the reticular dermis in which collagen fibers are thicker, more ordered, and sparsely populated with cells [1]. This complex nature of the skin makes it particularly difficult to replicate in the laboratory.
For many wounds, the healing process follows an ordered series of events including homeostasis, inflammation, proliferation/matrix deposition, and remodeling (reviewed in detail [1, 6]). For repair to occur, fibroblasts and other cells must fill the void created by the injury, with new blood vessels and ECM to form the granulation tissue, over which keratinocytes migrate to reseal the skin [6]. However, in cases such as burns where the damage to the epidermis and dermis can be extensive, the repair process is more complex. Here, cells and matrix to support the restoration of the skin are often reduced, or lacking, depending on the depth and severity of the injury. This leads not only to a slow healing process but also the potential for increased scar formation.
There is a vast number of treatments on the market for the management of wounds and burns [7], with the majority being wound dressings. Current wound dressings are comprised of a wide range of material types and claims with regard to what they treat. However, questions remain as to how well they facilitate the healing process [8]. Wound dressings, including films and foam dressings, are made from various materials, with some containing biologics or materials know to have antibacterial properties or agents that can facilitate cell migration. Additionally, there is a number of therapies currently on the market, such as skin substitutes derived from either de-epidermized tissue that can contain skin-derived cells, or alternatively cells, including fibroblasts and keratocytes, within a biological matrix or delivery vehicle [7], which will be described in more detail throughout the review.
Review
Wound dressings
Wound dressings have been fabricated out of different types of materials and various formats, for example fiber mats and hydrogels, and may contain additivities like silver for anti-bacterial properties. Conventional wound dressings serve to create a sealed wound environment to keep out infection, while also creating a moist environment to promote the wound healing process (Fig. 2). Recent progresses in the development of advanced wound dressings has seen the use of materials and/or the incorporation of biologics capable of either stimulating or promoting events in wound healing, from cellular migration, to the production of ECM components [9].
Fiber mats
Conventional wound dressings were originally made from cotton gauze or non-woven blends of similar materials. Current research into wound dressings includes electrospun mats that create a coverage for the wound but enable the exchange of gases through the dressing. Fiber mats prepared from polymers, including polycaprolactone, often include incorporation of a biological material like collagen [10] to mimic the dermis. Incorporation of known antibacterial compounds including silver [11] and gentamicin [12] are an added feature of many of these dressings.
One of the drawbacks of using synthetic materials, like polycaprolactone, as a wound dressing is that the dressing will eventually need to be removed, which may cause further damage to the wound. Fiber mats produced from natural materials, including dermal proteins, can be made to create wound dressings that mimic the ECM of the skin and can subsequently be incorporated into the body. Depending on the polymer/protein used, it may also stimulate wound healing responses. Fibronectin is one such protein found within the dermis and has been used to make scaffolds for potential wound healing therapies, which have been shown to not only accelerate wound healing but improve structural remodeling of the dermis and epidermis following healing [13]. The use of materials for the fabrication of scaffolds not only serves as material that biologically mimics the tissue that it is replacing, but it may also mimic the structure (Fig. 3).
Scanning electron micrographs (SEMs) of the micro- and macro-structure of a native dermal extracellular matrix (ECM) and b fibronectin scaffolds for wound healing applications. Figure adapted with permission from the original article of Chantre et al. [13]. (Copyright 2018 by Elsevier Ltd)
Hydrogels
Hydrogels (Fig. 4a) are good candidates for wound dressings as they are able to form a barrier from pathogens, as well as create a hydrated environment to help promote the body’s own wound healing response [14]. Poly(vinyl alcohol) (PVA) is a polymer that is commonly used in the fabrication of hydrogels and is frequently used in wound healing applications. PVA is often used in medical applications as it is known for its anti-protein fouling properties and is relatively biologically inert [15]. PVA hydrogels for wound healing often include other materials to stimulate the wound healing response such as curcumin [16] or zinc oxide nanoparticles [17] for antibacterial properties, and phlorotannins, derived from brown algae, which have been shown to promote fibroblast migration [18]. A polymer similar to PVA, poly(ethylene glycol) (PEG), is also commonly used for the fabrication of hydrogels, where Polymyxin B conjugated to PEG [46], however, if successful only a single graft is required.
Epidermal substitutes
As highly specialized epithelial cells, the epidermal keratinocytes provide skin with the ability to act as a barrier to the external environment and help prevent dehydration. Roughly 90% of the epidermis consists of keratinocytes, with the basal keratinocytes housing many of the keratinocyte stem cells that continuously replenish the skin with its new layers [2, 47, 48]. The basal stem cells divide and many of these cells differentiate, eventually losing their organelles as they are continually pushed up, by the newer dividing cells, so they form the outer most layer, the stratum corneum. Since the first successful keratinocyte culture in the 1970s, these cells have been used to treat burns, either as allografts or autografts. Traditionally, they were typically transferred to the burn site as sheets of cells, but these sheets are fragile, and therefore, substitutes, such as EpiCel™, that provide a more stable surface for their transfer have been developed. EpiCel™ (Fig. 5b) is formed by growing a sheet of autologous keratinocytes to two to eight cells thick on mouse 3T3 fibroblasts, which takes around 16 days, and then the sheet of keratinocytes is attached to a petroleum gauze. This is then layered onto the wound and the gauze is removed 7 days later. It is around 50 cm2 but can still suffer from fragility when relocating it to the wound.
Basal keratinocytes with their organelles intact are the main cell type responsible for the re-epithelialization process after injury and contain the stem cells responsible for regeneration [2, 48]. Recently, keratinocytes have been used in gene therapy to treat the skin disease epidermolysis bullosa, which like some burns can lead to wounds covering a large surface area [49]. Keratinocytes were genetically modified to contain the wild-type LAM3B (laminin 332) gene and grown as sheets of cells containing approximately 4% holoclones (the stem cells) [49]. These sheets of cells were shown to restore skin integrity over 80% of the body and correct the defect as defined by the presence of laminin 332 in skin with no blister formation observed 2 years later [49]. More importantly, they showed through polymerase chain reaction and clonal tracing that transient amplifying progenitors have a half-life of 3–4 months and the regenerated skin was sustained only by these long-lived stem cells (holoclones) [49]. This is good news for the use of cultured epithelial autografts as it confirms that, when grown correctly, cultured epithelial autographs can restore skin integrity and are incorporated into the skin for life. However, it should be noted that the patient’s dermis was intact, while for many burns patients the dermis is reduced or missing after injury, so presenting a further challenge that is driving research into develo** more epidermal/dermal substitutes.
Epidermal/dermal substitutes
During the normal wound healing process, there is continuous cross talk between keratinocytes in the epidermis and fibroblasts (and other cells) in the dermis [6]. This communication, in the form of mediators such as growth factors, co-ordinates actions that restore tissue [6]. This, along with the lack of a dermis in some burns, has led to skin substitutes being designed around scaffolds that contain both keratinocytes and fibroblasts [7, 45] (Fig. 6). The idea being to more closely mimic the normal skin architecture and the communication that occurs between the dermis and the epidermis in the substitutes.
Apligraf® is one such example of an epidermal/dermal substitute [7, 45]. It is constructed using neonatal dermal fibroblasts grown in a matrix that consists of bovine-derived type I collagen with layers of human neonatal epidermal keratinocytes on top that have been exposed to air to promote stratification in order to mimic the stratum corneum. This upper layer then acts as an effective barrier to the environment. Another similar bilayer cellular substitute is OrCel™ where neonatal fibroblasts are cultured on one side of a bovine-derived type I collagen sponge and keratinocytes on the other side [7, 45]. The matrix is absorbed during the healing process, and according to the manufacturer, DNA from the allogenic cells is no longer present 2–3 weeks after application.
Future directions
The heterogeneous nature of wounds, whether they are acute or chronic, the patients underlying pathologies, and the degree as to which the wound penetrates through the layers of the skin increase the complexity of develo** a therapy that is appropriate for all wounds. Where the therapies detailed in this review are typically developed for a specific wound type, for example, Novosorb™, a biodegradable synthetic polymer, has been developed for burn patients with full-thickness wounds to a significant percentage of their body surface area (~ 20–50%) [50], whereas Apligraf™, produced from bovine collagen and human-derived cells, is for the treatment of chronic venous leg ulcers and diabetic foot ulcers, and while the existing dressings and skin substitutes are good, they can be improved. The ECM, in addition to providing a scaffold for cells to adhere to and migrate on, provides mechanical stability and biochemical cues that play roles in tissue homeostasis and during the repair process [51]. It is comprised of over 300 proteins, 200 glycoproteins, and 30 proteoglycans, and so its exact composition, which can differ over time and under different circumstances, such as inflammation and after injury, can alter the outcome of the repair process. The ECM, and the growth factors housed within it, interacts with cells, triggering signaling pathways that can lead to proliferation, cell motility, or stasis depending on its composition. Our understanding of the composition of the ECM, and how the presence of specific combinations of proteoglycans can alter its structure and function, is relatively limited compared to what is known about the composition and formation of the epidermis. While there is no doubt that neonatal fibroblasts produce ECM that is beneficial to the repair process, whether the neonatal fibroblasts produce an ECM composition that is the “best” for wound healing or whether it can be fine-tuned to make the cells produce additional ECM components and growth factors that will improve the process is yet to be fully elucidated. One of the challenges that needs to be tackled is the ability to recreate the complexity of the dermis. The development of biomaterials going forward for wound healing therapies will need to approach these issues of creating an environment that closely resembles that of native skin, where materials in the future should mimic those present in the dermis in terms of their structure as well as biologic functionality. Current and future research will help answer these questions and aid the development of both dressings and skin substitutes to improve burn wound healing.
Along with the development of materials and technologies to more economically produce materials for wound healing therapies, technologies for the fabrication of scaffolds that use these materials have too advanced in recent years. The ability to manufacture scaffolds using 3D printing technologies has enabled the development of skin substitutes that not only can be produced to be specific to patient wounds but also the use of bioinks that allow the printing of scaffolds laden with cells [52]. Furthermore, advances in bioprinting and bioinks now enable the direct printing of scaffolds onto parts of the body, opening up the ability to print scaffolds directly onto patients wounds in the future [53]. Additionally, the ability to print scaffolds that can be fabricated to contain multiple layers consisting of different materials and laden with different cell types is a step towards being able to approach the challenge of creating the heterogenous structure of skin in the laboratory.
For burns patients, the ability to collect skin for autografts can be limited by the area of the burn and the sites that containing healthy skin. This has led to research into other sources of stem cells [2]. Hair follicles are easily accessed and contain stem cells capable of differentiating into and restoring skin after grafting [47]. EpiDex™ is an autologous epidermal equivalent generated from follicular stem cells (out root sheet cells) taken from patient’s hair. Stem cells from 50 to 200 hairs plucked from patients are cultured on a microporous membrane with fibroblast feeder layer of growth-arrested human dermal fibroblasts on the lower side. The cells are then detached from the microporous membrane and attached to a silicone membrane ready for use. The disadvantage here is the size of the EpiDex™, which is 1 cm2, making it unsuitable for large burns. Further research is needed to develop larger grafting material, incorporation of stem cells from different populations, or using induced pluripotent stem cells derived from blood cells that are reprogrammed back into an embryonic-like pluripotent state that permits these cells to then differentiate into keratinocytes or fibroblasts.
When the dermis and epidermis are lost due to a burn injury, some of the structures typically found in these areas are more often not replaced during the repair process. This includes hair follicles and sweat glands. This means that the skin that regenerates is generally hairless and does not sweat properly. No epidermal/dermal substitute has been developed yet that contains structures such as hair follicles or sweat glands. Also missing from scar tissue are melanocytes, the cells that produce pigments that give the skin its color. No skin substitutes to date contain these cells, but research in mice using skin substitutes containing melanocytes suggest that skin tone can be regained [54]. Incorporation of adipose-derived stem cells into a recombinant collagen scaffold demonstrated superior wound healing when compared to the recombinant protein scaffold alone [55]. The ability to incorporate stem cells that are able to differentiate into various lineages, depending on their environment, coupled with material scaffolds that are able to facilitate these environment ques, show enormous promise in their ability to facilitate wound healing and direct the next generation of wound healing therapies [56].
Conclusions
This review details a variety therapies that are currently available to patients for the treatment of wounds and burns that incorporate a biomaterial component. These therapies range from polymer hydrogels to epidermal/dermal substitutes that incorporate both keratinocytes and dermal fibroblasts. Due to the heterogeneous nature of wounds, there is no “one fits all” therapy, though the continual advancement in technologies used to develop these therapies, from 3D printing of dressings directly onto a wound, to stem cell technologies including induced pluripotent stem cells, will result in new wound healing therapies in the future.
Abbreviations
- ECM:
-
Extracellular matrix
- FGF:
-
Fibroblast growth factor
- PDGF:
-
Platelet-derived growth factor
- PEG:
-
Poly(ethylene glycol)
- PVA:
-
Poly(vinyl alcohol)
- TGFβ:
-
Transforming growth factor beta
- VEGF:
-
Vascular endothelial growth factor
References
Rognoni E, Watt FM. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol. 2018;28(9):709–22.
Chermnykh E, Kalabusheva E, Vorotelyak E. Extracellular matrix as a regulator of epidermal stem cell fate. Int J Mol Sci. 2018;19:1003.
Cole MA, Quan T, Voorhees JJ, Fisher GJ. Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J Cell Commun Signal. 2018;12:35–43.
Thulabandu V, Chen D, Atit RP. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev Dev Biol. 2018;7:e307.
Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–75.
Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017;18:1545.
Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci. 2017;18:789.
Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4:560–82.
Farrugia BL, Mi Y, Kim HN, Whitelock JM, Baker S, Wiesmann WP, et al. Chitosan-based heparan sulfate mimetics promote epidermal formation in a human organotypic skin model. Adv Funct Mater. 2018;28:1802818.
Ehterami A, Salehi M, Farzamfar S, Vaez A, Samadian H, Sahrapeyma H, et al. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int J Biol Macromol. 2018;117:601–9.
Tra Thanh N, Ho Hieu M, Tran Minh Phuong N, Do Bui Thuan T, Nguyen Thi Thu H, Thai VP, et al. Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater Sci Eng C. 2018;91:318–29.
Permyakova ES, Polčak J, Slukin PV, Ignatov SG, Gloushankova NA, Zajíčková L, et al. Antibacterial biocompatible PCL nanofibers modified by COOH-anhydride plasma polymers and gentamicin immobilization. Mater Des. 2018;153:60–70.
Chantre CO, Campbell PH, Golecki HM, Buganza AT, Capulli AK, Deravi LF, et al. Production-scale fibronectin nanofibers promote wound closure and tissue repair in a dermal mouse model. Biomaterials. 2018;166:96–108.
Kamoun EA, Kenawy ES, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res. 2017;8:217–33.
Barrett DA, Hartshorne MS, Hussain MA, Shaw PN, Davies MC. Resistance to nonspecific protein adsorption by poly(vinyl alcohol) thin films adsorbed to a poly(styrene) support matrix studied using surface plasmon resonance. Anal Chem. 2001;73:5232–9.
He F, Jiao H, Tian Y, Zhao L, Liao X, Fan Z, et al. Facile and large-scale synthesis of curcumin/PVA hydrogel: effectively kill bacteria and accelerate cutaneous wound healing in the rat. J Biomater Sci Polym Ed. 2018;29:325–43.
Khorasani MT, Joorabloo A, Moghaddam A, Shamsi H, MansooriMoghadam Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int J Biol Macromol. 2018;114:1203–15.
Park H-H, Ko S-C, Oh G-W, Heo S-J, Kang D-H, Bae S-Y, et al. Fabrication and characterization of phlorotannins/poly (vinyl alcohol) hydrogel for wound healing application. J Biomater Sci Polym Ed. 2018;29:972–83.
Lei W, **n L, Tianyu S, Yung-Hao T, Hong C, **aoyang X. Dual-functional dextran-PEG hydrogel as an antimicrobial biomedical material. Macromol Biosci. 2018;18:1700325.
Liao J, Jia Y, Wang B, Shi K, Qian Z. Injectable hybrid poly(ε-caprolactone)-b-poly(ethylene glycol)-b-poly(ε-caprolactone) porous microspheres/alginate hydrogel cross-linked by calcium gluconate crystals deposited in the pores of microspheres improved skin wound healing. ACS Biomater Sci Eng. 2018;4:1029–36.
Juhlin L. Hyaluronan in skin. J Intern Med. 1997;242:61–6.
Huang J, Ren J, Chen G, Li Z, Liu Y, Wang G, et al. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds. Mater Sci Eng C. 2018;89:213–22.
Birajdar MS, Halake KS, Lee J. Blood-clotting mimetic behavior of biocompatible microgels. J Ind Eng Chem. 2018;63:117–23.
Zhu J, Li F, Wang X, Yu J, Wu D. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl Mater Interfaces. 2018;10:13304–16.
Liyang S, Yannan Z, Qifan X, Caixia F, Jöns H, Jianwu D, et al. Moldable hyaluronan hydrogel enabled by dynamic metal–bisphosphonate coordination chemistry for wound healing. Adv Healthc Mater. 2018;7:1700973.
Zhu L, Bratlie KM. pH sensitive methacrylated chitosan hydrogels with tunable physical and chemical properties. Biochem Eng J. 2018;132:38–46.
Zhou Q, Kang H, Bielec M, Wu X, Cheng Q, Wei W, et al. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr Polym. 2018;197:292–304.
Tarusha L, Paoletti S, Travan A, Marsich E. Alginate membranes loaded with hyaluronic acid and silver nanoparticles to foster tissue healing and to control bacterial contamination of non-healing wounds. J Mater Sci Mater Med. 2018;29:22.
Liu R, Dai L, Si C, Zeng Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr Polym. 2018;195:63–70.
Kreuger J, Spillmann D, Li J-p, Lindahl U. Interactions between heparan sulfate and proteins: the concept of specificity. J Cell Biol. 2006;174:323–7.
Farrugia B, Lord M, Melrose J, Whitelock J. The role of heparan sulfate in inflammation, and the development of biomimetics as anti-inflammatory strategies. J Histochem Cytochem. 2018;66(4):321–36 Accepted 10 Oct 2017.
Wu J, Ye J, Zhu J, **ao Z, He C, Shi H, et al. Heparin-based coacervate of FGF2 improves dermal regeneration by asserting a synergistic role with cell proliferation and endogenous facilitated VEGF for cutaneous wound healing. Biomacromolecules. 2016;17:2168–77.
Watarai A, Schirmer L, Thönes S, Freudenberg U, Werner C, Simon JC, et al. TGFβ functionalized starPEG-heparin hydrogels modulate human dermal fibroblast growth and differentiation. Acta Biomater. 2015;25:65–75.
Yili Q, Cong C, Qingqing W, Ai H, Ying S, Hongling L, et al. The dual delivery of KGF and bFGF by collagen membrane to promote skin wound healing. J Tissue Eng Regen Med. 2018;12:1508–18.
Bienert M, Hoss M, Bartneck M, Weinandy S, Böbel M, Jockenhövel S, et al. Growth factor-functionalized silk membranes support wound healing in vitro. Biomed Mater. 2017;12:045023.
Leonard A, Koria P. Growth factor functionalized biomaterial for drug delivery and tissue regeneration. J Bioact Compat Polym. 2017;32:568–81.
Ouyang Q-Q, Hu Z, Lin Z-P, Quan W-Y, Deng Y-F, Li S-D, et al. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int J Biol Macromol. 2018;112:1191–8.
Avila Rodríguez MI, Rodríguez Barroso LG, Sánchez ML. Collagen: a review on its sources and potential cosmetic applications. J Cosmet Dermatol. 2018;17:20–6.
Yang C, Hillas PJ, Baez JA, Nokelainen M, Balan J, Tang J, et al. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18:103–19.
Darby IA, Laverdet B, Bonte F, Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–11.
Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229:298–309.
Mine S, Fortunel NO, Pageon H, Asselineau D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PLoS One. 2008;3:e4066.
Jahoda CA, Reynolds AJ. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet. 2001;358:1445–8.
Neuman MG, Nanau RM, Oruna-Sanchez L, Coto G. Hyaluronic acid and wound healing. J Pharm Pharm Sci. 2015;18:53–60.
Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7:229–58.
Fetterolf DE, Istwan NB, Stanziano GJ. An evaluation of healing metrics associated with commonly used advanced wound care products for the treatment of chronic diabetic foot ulcers. Managed Care. 2014;23(7):31–8.
Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–56.
Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci. 2015;16:25476–501.
Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327–32.
Greenwood JE, Schmitt BJ, Wagstaff MJD. Experience with a synthetic bilayer biodegradable temporising matrix in significant burn injury. Burns Open. 2018;2:17–34.
Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–12.
van Kogelenberg S, Yue Z, Dinoro JN, Baker CS, Wallace GG. Three-dimensional printing and cell therapy for wound repair. Adv Wound Care. 2018;7:145–56.
Zhu Z, Guo SZ, Hirdler T, Eide C, Fan X, Tolar J, et al. 3D printed functional and biological materials on moving freeform surfaces. Adv Mater. 2018;30:e1707495.
Boyce ST, Lloyd CM, Kleiner MC, Swope VB, Abdel-Malek Z, Supp DM. Restoration of cutaneous pigmentation by transplantation to mice of isogeneic human melanocytes in dermal–epidermal engineered skin substitutes. Pigment Cell Melanoma Res. 2017;30:531–40.
Mashiko T, Takada H, Wu S-H, Kanayama K, Feng J, Tashiro K, et al. Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. J Tissue Eng Regen Med. 2018;12:1186–94.
Teixeira CM, Pedro PR, Pinto MA. Stem cells in skin wound healing: are we there yet? Adv Wound Care. 2016;5:164–75.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the contents and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Murray, R.Z., West, Z.E., Cowin, A.J. et al. Development and use of biomaterials as wound healing therapies. Burn Trauma 7, 2 (2019). https://doi.org/10.1186/s41038-018-0139-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41038-018-0139-7