Abstract
Capillary leak syndrome (CLS) represents a phenotype of increased fluid extravasation, resulting in intravascular hypovolemia, extravascular edema formation and ultimately hypoperfusion. While endothelial permeability is an evolutionary preserved physiological process needed to sustain life, excessive fluid leak—often caused by systemic inflammation—can have detrimental effects on patients’ outcomes. This article delves into the current understanding of CLS pathophysiology, diagnosis and potential treatments. Systemic inflammation leading to a compromise of endothelial cell interactions through various signaling cues (e.g., the angiopoietin–Tie2 pathway), and shedding of the glycocalyx collectively contribute to the manifestation of CLS. Capillary permeability subsequently leads to the seepage of protein-rich fluid into the interstitial space. Recent insights into the importance of the sub-glycocalyx space and preserving lymphatic flow are highlighted for an in-depth understanding. While no established diagnostic criteria exist and CLS is frequently diagnosed by clinical characteristics only, we highlight more objective serological and (non)-invasive measurements that hint towards a CLS phenotype. While currently available treatment options are limited, we further review understanding of fluid resuscitation and experimental approaches to target endothelial permeability. Despite the improved understanding of CLS pathophysiology, efforts are needed to develop uniform diagnostic criteria, associate clinical consequences to these criteria, and delineate treatment options.
Graphical Abstract

Similar content being viewed by others
Introduction
Capillary leak syndrome (CLS) refers to a syndrome of deranged fluid homeostasis, often observed in critically ill [1,2,3]. In clinical practice, CLS is frequently defined by excessive fluid shift from the intravascular to the extravascular space, resulting in intravascular hypovolemia, extravascular edema formation, and hypoperfusion—necessitating further fluid resuscitation [3].
In health, fluid exchange between intravascular and extravascular spaces is vital for maintaining the body’s homeostasis. However, disturbances in this delicate equilibrium, often driven by systemic inflammation (e.g., sepsis) can lead to the clinical picture of CLS [4,5,6].
Despite efforts to define CLS, there is no established clinical definition nor accepted diagnostic criteria [1, 3]. Previously, Marx et al. characterized CLS as a fluid extravasation, resulting in edema and hypoperfusion [3]. The authors studied septic shock patients using various methods such as indocyanine green measurements, chromium-51 labeled erythrocytes, and colloid osmotic pressure measurements, aiming to differentiate CLS from other hypo-oncotic conditions and clinical scenarios associated with fluid retention [3]. The definition of CLS proposed by Marx et al. in 2000 emphasized three main aspects: intravascular hypovolemia despite fluid resuscitation, generalized edema, and hemodynamic instability. This pivotal description, while not universally adopted, offers valuable insight into the key features of CLS, aiding in the differentiation of this syndrome from other conditions that share similar clinical manifestations. It underlines the necessity for an accepted definition of this syndrome to develop targeted and effective therapeutic interventions [3].
This article will review the current understanding adding new aspects of CLS in clinical practice, and give an overview about the pathophysiology, clinical presentation, diagnostic and therapeutic options.
Pathophysiology of CLS and implications
Triggers and key features
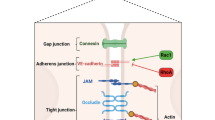
A CLS phenotype can be triggered by numerous disease states as well as certain medications and toxins [7]. Depending on the literature source, terms like “generalized hyperpermeability syndrome”, “endothelial permeability” or “capillary leakage” may be used synonymously for CLS. As an important semantic distinction, the “idiopathic systemic capillary leak syndrome”, also referred as Clarkson’s disease [114,115,116].
The destructive process of glycocalyx during sepsis has substantial physiological implications. The loss of this protective barrier directly impacts local tissue, but the degradation products themselves can also circulate and affect distant sites in the body [85]. This leads to a system-wide impact that contributes to fluid shifts and the multiple organ dysfunction often seen in septic patients. The extent and the specific mechanisms through which glycocalyx degradation affects the progression and prognosis of sepsis are still being uncovered. This understanding is critical for the development of therapeutic strategies to preserve the eGC, attenuate the inflammatory response, and ultimately improve the outcomes of sepsis.
Fluid overload and dynamics
The human body contains various fluid compartments, both intravascular and extravascular, which have specific volumes and protein contents. According to indicator dilution studies, a healthy 70 kg adult typically has about 3 L of plasma, containing around 210 g of protein [117]. On the other hand, the same adult will have approximately 12 L of interstitial fluid. This fluid resides in a gel phase and contains 240–360 g of protein. The capillary pressure in this system is higher than the pressure in the interstitial space, which drives the movement of the solvent and its small lipophobic solutes towards the interstitial space [118]. Trans-endothelial fluid shifts are regulated by the vascular barrier in addition to hydrostatic and oncotic forces, as described by the revised Starling equation [119]. In healthy organs, the increased permeability and movement of proteins and plasma fluid are temporary and decrease once the stimulating factor is removed. Edema is traditionally perceived as a consequence of a pressure-driven net outward filtration in the capillary, partially reversed by fluid reabsorption at the venous end by an oncotic pressure gradient [120]. Contrary to traditional perspectives, more recent theories propose that continuous net filtration is the norm in most capillary networks [121]. Apart from an increased pressure gradient, edema can also be caused by hypo-oncotic states, changes in permeability and impaired lympathics.
The capillary wall includes a glycocalyx layer, which is a complex meshwork of glycosaminoglycans and additional glycoproteins. This layer serves as a filtration barrier, featuring gaps where capillary filtration takes place [121,122,123]. The movement occurs through regulation of the glycocalyx and the occasional breaks in the inter-endothelial junctions. These breaks constitute less than 0.1% of the total endothelial surface area, allowing a highly regulated fluid exchange process [117]. The glycocalyx layer was previously assumed to have an almost perfect reflection coefficient for proteins, particularly albumin. However, albumin diffusion through capillary pores results in about half of the body's albumin content residing extravascularly, with interstitial oncotic pressure reaching 30–60% of plasma oncotic pressure [124]. The complexity of the interstitial space has been underestimated in the past. It actually consists of a triphasic system that includes freely moving fluids, a gel-like phase rich in large polyanionic glycosaminoglycans molecules, and a collagen framework [117, 124]. Albumin is predominantly absent from this luminal surface, leading to a stronger intravascular oncotic pressure than what direct measurements of interstitial albumin concentration would suggest [125]. As a result, the net filtration process is more influenced by the oncotic pressure beneath the endothelial glycocalyx than by the capillary membrane itself [123].
The clinical consequences of these fluid shifts can be manifold, yet not immediately visible to the clinician. The lungs are especially prone to pulmonary edema due to the unfavorable ratio of endothelium per tissue with the clinical potential to impact gas exchange, and predispose the lungs to further infectious complications [126]. Additionally, the gastrointestinal tract may become edematous, leading to paralytic ileus, an increase in intra-abdominal pressure and subsequent tissue hypoxia, and impaired wound healing [127, 128]. It is noteworthy that the endothelium is highly heterogeneous across different vascular beds; for example, CLS commonly affects various organs but is rarely observed in the brain due to the unique properties of the blood–brain barrier, including a higher pericyte-to-endothelial cell ratio that contributes to its greater impermeability [129].
While CLS is widely acknowledged in the critical care settings, there is a surprising lack of clinical studies exploring its impact on organ dysfunction and mortality [1]. This may stem from the current absence of accepted diagnostic criteria for CLS. However, associated conditions like an inflammatory state and positive fluid balance—circumstances inevitably related to CLS—correlate with higher mortality rates in the ICU [130]. For example, elevated levels of serum cytokines are commonly observed in non-survivors of critically illness, and a positive fluid balance is acknowledged as an independent predictor of outcomes in patients with sepsis [131, 132].
Fluid management can be complex in ICU settings, demanding a thorough understanding of body fluid homeostasis [133]. Fluid overload, which comprises whole body water, i.e., extra- and intravascular fluid, can be detrimental and associated with negative outcomes in patients who are critically ill [134,135,136,137,138,139,140,141,142,143,144,3).
Preservation of the endothelial surface layer (ESL)
In this section, the term 'Endothelial Surface Layer (ESL)' will be used to refer to the intricate structure formed by the endothelial glycocalyx (eGC) along with associated plasma proteins. The eGC serves as a luminal mesh that provides endothelial cells with a framework to bind plasma proteins and soluble glycosaminoglycans [181]. While the eGC itself is considered inactive, it becomes physiologically active once it binds with or is immersed in plasma constituents, thereby forming the ESL. It is worth noting that the specific roles and clinical relevance of the eGC as part of the broader ESL are subjects of ongoing research. The ESL is instrumental in maintaining vascular homeostasis, regulating vascular permeability, and acting as a mechanosensor for hemodynamic shear stresses, in addition to displaying antithrombotic and anti-inflammatory characteristics [182]. Plasma proteins, especially albumin, bind within the glycocalyx and aid in stabilizing this layer [183]. Albumin's function is particularly important as it contributes to plasma colloid osmotic pressure (among other, often unmeasured molecules). Moreover, albumin performs a range of roles—from acting as a free radical scavenger and transporting sphingosine-1-phosphate (which has protective effects on the endothelium), to providing immunomodulatory and anti-inflammatory effects [125].
Experimental studies have highlighted the multifunctional nature of albumin, which includes maintaining ESL integrity, partially restoring compromised vascular permeability, exhibiting anti-oxidative properties and anti-inflammatory properties, improving hemodynamics and microcirculation following endotoxemia or hemorrhagic shock, and acting as an effective plasma volume expander [125, 184,185,186,187,188,189,190]. Interestingly, beneficial effects appear to be independent of albumin's oncotic properties. Additional research has shown that the choice of fluid for infusion significantly affects the ESL [125, 191]. For instance, in vivo experiments conducted on anesthetized rats subjected to hemorrhagic shock followed by fluid resuscitation, the use of normal saline failed to restore ESL thickness and plasma levels of syndecan-1 [192]. Conversely, albumin was found to stabilize permeability and leukocyte rolling/adhesion, partially restoring ESL thickness and reducing plasma syndecan-1 to baseline levels [125, 192]. Authors have proposed several mechanisms to elucidate the positive influence of albumin on the endothelium [193]. Primarily, albumin might alleviate sepsis-induced damage to the ESL. As reviewed by Aldecoa et al., albumin, due to its amphoteric properties, has the ability to establish strong bonds with the ESL, while its negative charge aids in maintaining its parietal electrical barrier [125]. In addition, the antioxidant functions of albumin are well-documented [125]. Albumin's free thiol group, carried by a cysteine residue (Cys-34), assists in neutralizing harmful plasma free radicals, which is highly relevant in the septic environment marked by a high oxidative state. Lastly, albumin's capacity to form complexes with heavy metals provides protection against oxidation via the Fenton reaction [193]. Hariri et al. underscore the mounting evidence, both from experimental models and in the context of critically ill patients, that suggests the protective role of albumin on the endothelium during acute injury [193]. Preservation of the ESL using albumin (and fresh frozen plasma) is intriguing, however clinical studies need to confirm these findings. It is anticipated that the ongoing multicenter ARISS trial will further shed light into the effects of albumin on clinical outcomes [194]. It is crucial to note that commercial albumin solutions are often heated to 60 °C for several hours for inactivation of infectious agents [195]. This heat treatment can lead to protein denaturation and alterations in its negative charge [195], raising the question of the comparability of administered albumin with physiologically circulating albumin synthesized by the liver.
Various clinical studies examine the effects of albumin in the clinical context. Zdolsek et al. have shed light on the impact of exogenous albumin administration on fluid dynamics under various clinical conditions [196]. The primary focus of their study was to evaluate the rate at which infused albumin dissipates from the bloodstream, quantified as the half-life (T1/2), under different clinical scenarios. Their research involved intravenously infusing 3 mL/kg of 20% albumin into a varied population that included healthy volunteers, patients after burns, postoperative patients, and patients who underwent surgery with both minor and significant bleeding. The results showed a consistent T1/2 across all groups, except for those who experienced surgery with major bleeding. In the latter case, the infused albumin disappeared faster, indicating a greater loss of albumin in situations of significant hemorrhage. Zdolsek and colleagues further compared the effects of 20% and 5% albumin concentrations on plasma volume expansion [197]. The study was designed in a way that the same mass of albumin was administered under both scenarios. Their findings showed that while both concentrations led to plasma volume expansion, the 5% albumin concentration had a slightly higher rate of volume expansion. However, they found that a third of the 5% albumin solution quickly leaked from the plasma, likely due to the higher colloid osmotic pressure of volunteer plasma than that of the albumin solution. By the 6-h mark, about 42–47% of the administered albumin had leaked from the capillaries, regardless of the concentration used.
Further research by Hahn and colleagues investigated the body fluid shifts when 20% albumin is administered intravenously, with a specific focus on postoperative patients [198]. They found that the infused albumin expanded the plasma volume beyond the volume of the infusion itself by moving non-circulating fluid. However, the same mechanism also increased fluid losses from the system. Despite these dynamics, they observed that the plasma albumin level and plasma volume remained stable for about 2 h post-infusion. Therefore, the effectiveness of albumin as an administered fluid may depend on the specific clinical scenario and the administered concentration.
Microvascular and ESL protection prior to surgeries (i.e., before an anticipated inflammatory insult) presents an interesting area of research, as highlighted by Yanase et al. [199]. In their study, they explored the feasibility, efficacy, and safety of potential protective influence of dexamethasone and albumin on the ESL in patients undergoing abdominal surgery. In this trial, patients were randomly assigned to two groups. One group was given intravenous dexamethasone and 20% albumin at the onset of anesthesia, followed by additional albumin with each subsequent crystalloid administration. The control group, conversely, received only crystalloid fluid without dexamethasone leading to differences in the crystalloid, colloid administration. The outcomes were evaluated based on alterations in plasma syndecan-1 and heparan sulfate levels as markers for eGC damage, and inflammatory markers measured at four perioperative timepoints. Although no significant differences were noted in syndecan-1 levels between the two groups, the group that received the dexamethasone-albumin treatment demonstrated lower heparan sulfate and C-reactive protein levels on the first postoperative day, suggesting a potential protective effect on the glycocalyx. This group also experienced fewer postoperative complications [199]. It remains uncertain if this effect is related to the dexamethasone or albumin administration, or the combination thereof.
It has to be noted that the role of albumin administration in critically ill patients has been studied extensively in the past. The ALBIOS trial conducted by Caironi et al. [200] aimed to evaluate the efficacy of albumin administration in patients with severe sepsis. In this multicenter, open-label trial, 1818 patients with severe sepsis were randomized to receive either a 20% albumin and crystalloid solution or a crystalloid solution alone. The albumin group was targeted to maintain a serum albumin concentration of 30 g per liter or more until discharge from the ICU or 28 days after randomization. During the first 7 days, the albumin group demonstrated a higher mean arterial pressure and a lower net fluid balance compared to the crystalloid group. However, no significant difference was observed in the total daily amount of administered fluid between the two groups. The 28-day and 90-day mortality rates did not show significant differences between the two groups, indicating that albumin replacement in addition to crystalloids did not improve survival rates at these timepoints [200]. These findings do not support the hypothesis that albumin administration has survival benefits in severe sepsis, despite previous studies and experimental evidence for its protective role. However, the ALBIOS trial did confirm some physiological benefits of albumin administration. Patients in the albumin group exhibited superior hemodynamic responses, with a higher mean arterial pressure, lower heart rate, and lower net fluid balance in the first 7 days of treatment [200]. The average cardiovascular SOFA subscore was lower in the albumin group, and the time to suspension of inotropic or vasopressor agents was shorter, suggesting a decreased need for vasopressors [200]. Similar to the ALBIOS trial, the ALBICS trial for albumin use in cardiac surgery did not show a benefit on major adverse events at 90 days [201]. Many unanswered questions remain around the role of albumin administration, e.g., its role in effective de-resuscitation and augmenting loop diuretic effects [202] and the comparability of exogenously administered albumin’s properties compared to that of circulating albumin. Due to these reasons, no final recommendation can be given for the role of albumin administration for CLS treatment.
Lymphatics in ICU patients
Unlike the cardiovascular system, which ensures bidirectional blood flow, the lymphatic system is specifically designed for unidirectional transit from the extracellular space to the venous system [45]. The lymphatic system plays a pivotal role, actively participating in maintaining tissue fluid equilibrium, aiding in the absorption of lipids from the gastrointestinal tract, and playing an important role in the immune response by transporting antigen-presenting cells and lymphocytes to lymphoid organs [203]. Of note, the lymphatic flow can be increased in health and disease. In the context of critical care, the lymphatic system's potential for increased flow offers interesting avenues for research.
In the critical care setting, physical therapy involving manual lymphatic drainage presents an interesting approach as it has been shown to enhance lymphatic outflow and mobilize fluid [204,205,206]. Studies have found that manual lymphatic drainage can significantly improve the transportation of various substances within the lymphatic system [204,205,206,207]. The findings indicated that manual lymphatic drainage can lead to a modest increase in plasma volume, averaging around 1.5 ± 0.8% [207]. This expansion suggests that lymphatic fluid is being mobilized into the bloodstream. Recent research showed an increase in albumin levels following manual lymphatic drainage [207]. These changes were not solely due to fluid shifts, as albumin concentrations were corrected for changes in plasma volume, and hematocrit remained unaffected by the lymphatic drainage. These observations could imply that the mobilized fluid entering the bloodstream after manual lymphatic drainage therapy possesses a higher albumin content than plasma. The long-term implications of these physiological changes are yet to be fully understood. Nonetheless, the potential role of manual lymphatic drainage in influencing fluid balance and lymphatic outflow could have relevant implications for managing conditions in the ICU.
Experimental approach for endothelial stabilization
Phosphodiesterase (PDE) inhibitors exhibit a diverse range of pharmacological effects, encompassing properties such as anti-inflammatory, antioxidant, vasodilatory, cardiotonic, and anticancer activities, alongside enhancing memory. This expansive superfamily of PDEs is categorized into 11 distinct groups, differentiated by their structural characteristics, cellular localization, gene expression patterns, protein attributes, and a variety of pharmacological properties, influenced by both internal and external regulatory factors. Particularly, phosphodiesterase-4 inhibitors (PDE4-Is, e.g., rolipram and roflumilast) have been explored as potential treatment options stabilizing endothelial interaction during systemic inflammation and sepsis [208, 209]. The proposed mechanism is thought to involve the control of the cAMP/Rac1-signaling pathway, which is integral to the stability of intercellular junctions [208, 210,211,212]. The intracellular second messenger cyclic adenosine monophosphate (cAMP) decreases in endothelial cells under inflammatory conditions, associated with the breakdown of endothelial barrier properties in vitro [210]. Experimental studies further suggest that administration of PDE4-Is which increases endothelium-specific cAMP holds the potential to maintain cellular adhesion and endothelial barrier properties during acute inflammation. Schick et al. showed in a rodent model that the application of rolipram or roflumilast effectively attenuated capillary leakage and improved microcirculatory flow by preventing the inflammation-induced loss of endothelial cAMP [208]. Wollborn et al. further confirmed the effects of PDE4-Is in extracorporeal circulation-induced capillary leak [209]. Various other pathways remain under investigation to evaluate means to stabilize vascular endothelium [39].
In addition to PDE4-Is, other PDE-Is also show potential in endothelial stabilization. The PDE1 family, known for its vasodilatory effects and reduced activity in platelet aggregation, may influence endothelial stability by modifying vascular tone and cellular cAMP levels, crucial factors in maintaining endothelial barrier integrity [213,214,215]. Experimental studies suggest that PDE1 inhibitors, by modulating cGMP and cAMP pathways, could potentially reinforce endothelial cell adhesion and barrier properties, similar to the effects observed with PDE4-Is [216,217,218]. PDE2-Is, through their unique mechanism of cGMP-mediated cAMP regulation, may also contribute to endothelial stability. By enhancing intercellular communication and barrier function, they could offer a novel approach to managing endothelial disruption in conditions such as pulmonary hypertension and heart failure [219,220,221]. Furthermore, PDE3-Is, while primarily recognized for their cardiac effects, could indirectly influence endothelial function. Given their role in modulating intracellular cAMP levels, they might impact endothelial cell junction stability and barrier properties, particularly under stress conditions such as sepsis or systemic inflammation [222,223,224]. Among the most promising for endothelial stabilization are PDE5-Is like sildenafil and tadalafil. These agents have shown effectiveness in improving hemodynamics and endothelial function in heart failure and pulmonary arterial hypertension [225,226,227]. Their mechanism, which involves modulating cGMP-dependent signaling, makes them particularly relevant for maintaining endothelial barrier integrity. While primarily associated with visual functions, the role of PDE6 in other cellular processes remains under-investigated in the context of endothelial stabilization [228], PDE7-Is are present in immune cells and cardiac myocytes and might influence endothelial function indirectly through immunomodulatory pathways [229, 230]. Both PDE8 and PDE9 are involved in cAMP and cGMP signaling, respectively. While their direct role in endothelial stabilization is not as prominent, they may offer insights into cardiovascular functions and pathologies [231,232,233]. PDE10 and PDE11 are primarily explored for neurological and psychiatric disorders, and tumor development. Their role in endothelial stabilization is less defined [234, 235].
Recent insights have highlighted the pivotal role of vasodilators, particularly prostaglandins, in regulating endothelial capillary permeability. Prostaglandins, notably prostaglandin E2, play a significant role in this regard. Activation of the prostaglandin E2 receptor signal, which induces vasodilation, could be targeted to enhance endothelial barrier function and counteract capillary leak syndrome [236]. Experimental strategies might involve modulating these pathways to optimize vascular tone and permeability. Endothelium-derived vasodilators, including NO, prostacyclin, and endothelium-derived hyperpolarizing factors, play a central role in maintaining vascular tone. NO, synthesized by endothelial nitric oxide synthase, is instrumental in regulating vascular tone and endothelial function [237,238,239]. For example, strategies that enhance endothelial nitric oxide synthase activity or NO bioavailability could effectively stabilize endothelial function. This might include gene therapy to upregulate endothelial nitric oxide synthase expression, pharmacological agents to increase NO production, or novel compounds to mimic NO’s vasodilatory effects [236]. Additionally, addressing endothelial hyperpolarization through endothelium-derived hyperpolarizing factors could offer a novel experimental avenue. This might involve manipulating calcium-activated potassium channels or exploring the roles of gap junctions and epoxyeicosatrienoic acids in endothelial cell signaling [240, 241]. Prostacyclin, generated by cyclooxygenase in endothelial cells, activates adenylate cyclase, leading to vascular smooth muscle relaxation [242]. Its role in vasorelaxation suggests potential therapeutic applications in managing endothelial dysfunction. Modulating prostacyclin levels or mimicking its action through pharmacological agents could be an experimental approach to stabilize endothelial cells and maintain vascular homeostasis [236].
Recently, therapeutic plasma exchange has been used in clinical trials to modulate the injurious endothelial activation. The rationale behind this combines two aspects in one procedure: the removal of injurious circulating factors (e.g., Ang-2, heparanase-1) and the replacement of protective factors that have been consumed by the disease process (e.g., heparanase-2 or Ang-1) [243]. This concept has been demonstrated both by quantifying these factors before and after and by ex vivo stimulation of endothelial cells with plasma from these patients [95, 244, 245].
Conclusion
This review elucidates the multifaceted nature of CLS, underscoring the importance of recognizing its diverse triggers, including systemic inflammation and endothelial barrier breakdown. While current diagnostic methods, such as bioelectrical impedance analysis and serum markers, provide insights, their limitations highlight the need for more precise and universally accepted diagnostic criteria. Treatment strategies, primarily focusing on fluid management and endothelial stabilization, have shown potential, yet they lack specificity and efficacy for CLS. Innovative approaches, like the exploitation of the angiopoietin–Tie2 signaling axis, preservation of the endothelial surface layer, and experimental therapies like phosphodiesterase inhibitors, offer promising directions. Future research should aim to develop a consensus on CLS definition, establish reliable diagnostic benchmarks, and explore these novel therapeutic strategies to enhance patient outcomes in critical care settings.
Take-home message
CLS presents a diagnostic and therapeutic challenge in critical care due to its complex pathophysiology and the absence of standardized diagnostic criteria. According to the authors of this review, prioritizing research to refine diagnostic tools and explore novel treatments, including endothelial stabilization strategies and experimental pharmacological interventions, is crucial for improving patient management and outcomes in CLS.
Availability of data and materials
Not applicable.
References
Wollborn J, Hassenzahl LO, Reker D, Staehle HF, Omlor AM, Baar W (2021) Diagnosing capillary leak in critically ill patients: development of an innovative scoring instrument for non-invasive detection. Ann Intensive Care 11:175
Bichon A, Bourenne J, Gainnier M, Carvelli J (2021) Capillary leak syndrome: state of the art in 2021. Rev Med Interne 42:789–796. https://doi.org/10.1016/j.revmed.2021.05.012
Marx G, Vangerow B, Burczyk C, Gratz KF, Maassen N, Meyer MC (2000) Evaluation of noninvasive determinants for capillary leakage syndrome in septic shock patients. Intensiv Care Med 26:1252–1258
Kundra P, Goswami S (2019) Endothelial glycocalyx: role in body fluid homeostasis and fluid management. Indian J Anaesth 63:6–14
Cordemans C, Laet ID, Regenmortel NV, Schoonheydt K, Dits H, Huber W (2012) Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care 2:1
Lee WL, Slutsky AS (2010) Sepsis and endothelial permeability. N Engl J Med 363:689–691
Siddall E, K M and Radhakrishnan, J, (2017) Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int 92:37–46
**e Z, Ghosh CC, Patel R (2012) Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome. Blood 119:4321–4332
Druey KM (2010) Narrative review: the systemic capillary leak syndrome. Ann Intern Med 153:90–98
Wollborn J, Zhang Z, Gaa J et al (2023) Angiopoietin-2 is associated with capillary leak and predicts complications after cardiac surgery. Ann Intensive Care 13:70. https://doi.org/10.1186/s13613-023-01165-2
Schenker JG (1993) Prevention and treatment of ovarian hyperstimulation. Hum Reprod 8:653–659
Whelan JG III, Vlahos NF (2000) The ovarian hyperstimulation syndrome. Fertil Steril 73:883–896
Ramos-Casals M, Pilar B-Z, Lopez-Guillermo A (2014) Adult haemophagocytic syndrome. Lancet 383:1503–1516
Aulagnon F, Lapidus N, Canet E (2015) Acute kidney injury in adults with hemophagocytic lymphohistiocytosis. Am J Kidney Dis 65:851–859
Giles RB, Sheedy JA, Ekman CN (1954) The sequelae of epidemic hemorrhagic fever; with a note on causes of death. Am J Med 16:629–638
Peters CJ, Simpson GL, Levy H (1999) Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu Rev Med 50:531–545
Natterer J, Perez MH, Bernardo S (2012) Capillary leak leading to shock in Kawasaki disease without myocardial dysfunction. Cardiol Young 22:349–352
Prete M, Urso L, Fatone MC (2016) Antiphospholipids syndrome complicated by a systemic capillary leak-like syndrome treated with steroids and intravenous immunoglobulins: a case report. Medicine (Baltimore) 95:e2648
Guffroy A, Dervieux B, Gravier S, Martinez C, Deibener-Kaminsky J, Hachulla E (2017) Systemic capillary leak syndrome and autoimmune diseases: a case series. Semin Arthritis Rheum Februar 46:509–512
Chugh KS (1989) Snake-bite-induced acute renal failure in India. Kidney Int 35:891–907
Suchithra N, Pappachan JM, Sujathan P (2008) Snakebite envenoming in Kerala, South India: clinical profile and factors involved in adverse outcomes. Emerg Med J 25:200–204
Bradberry SM, Dickers KJ, Rice P (2003) Ricin poisoning. Toxicol Rev 22:65–70
Rosenberg SA, Lotze MT, Muul LM (1985) Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313:1485–1492
Winkler U, Jensen M, Manzke O (1999) Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 94:2217–2224
Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL (2011) Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med 3(88):25
Duchesne JC, Kaplan LJ, Balogh ZJ, Malbrain ML (2014) Role of permissive hypotension, hypertonic resuscitation and the global increased permeability syndrome in patients with severe hemorrhage: adjuncts to damage control resuscitation to prevent intra-abdominal hypertension. Anaesthesiol Intensiv Ther 47:143–155
Cotton BA, Guy JS, Morris JA, Abumrad NN (2006) The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock 26:115–121
Ince C (2015) Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 19(Suppl 3):S8. https://doi.org/10.1186/cc14726
Augustin HG, Kozian DH, Johnson RC (1994) Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes. BioEssays 16:901–906. https://doi.org/10.1002/bies.950161208
Ait-Oufella H, Maury E, Lehoux S, Guidet B, Offenstadt G (2010) The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med 36:1286–1298
Claesson-Welsh L, Dejana E, McDonald DM (2021) Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol Med 27:314–331
Duan C-Y, Zhang J, Wu H-L et al (2017) Regulatory mechanisms, prophylaxis and treatment of vascular leakage following severe trauma and shock. Military Med Res 4:11. https://doi.org/10.1186/s40779-017-0117-6
Mehta D, Malik AB (2006) Signaling mechanisms regulating endothelial permeability. Physiol Rev 86:279–367
Palade GE, Simionescu M, Simionescu N (1979) Structural aspects of the permeability of the microvascular endothelium. Acta Physiol Scand Suppl 463:11–32
Simionescu M (1984) Ultrastructure of the microvascular wall: functional correlations. In: Handbook of physiology, Section “Pathophysiology of CLS and implications” “Introduction”:Chap. 3
Bearer EL, Orci L (1985) Endothelial fenestral diaphragms: a quick-freeze, deep-etch study. J Cell Biol 100:418–428
Aird WC (2007) Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100:158–173
Wettschureck N, Strillic B, Offermanns S (2019) Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol Rev 99:1467–1525
Hellenthal KEM, Brabenec L, Wagner N-M (2022) Regulation and dysregulation of endothelial permeability during systemic inflammation. Cells 11:1935. https://doi.org/10.3390/cells11121935
Raia L, Zafrani L (2022) Endothelial activation and microcirculatory disorders in sepsis. Front Med 3:907992
Milam KE, Parikh SM (2015) The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation. Tissue Barriers 3:e757508
Vestweber D (2015) How leukocytes cross the vascular endothelium. Nat Rev Immunol 15:692–704
Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7:678–689
Hinsbergh VWM, Collen A, Koolwijk P (2006) Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci 936:426–437
Cueni LN, Detmar M (2008) The lymphatic system in health and disease. Lymphat Res Biol 6:109–122
Kubicki R, Grohmann J, Siepe M, Benk C, Humburger F, Rensing-Ehl A (2013) Early prediction of capillary leak syndrome in infants after cardiopulmonary bypass. Eur J Cardio-Thorac Surg 44:275–281
An R, Pang QY, Liu H (2019) Association of intra-operative hypotension with acute kidney injury, myocardial injury and mortality in non-cardiac surgery: a meta-analysis. Int J Clin Pract 73:13394
Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS (2019) Acute respiratory distress syndrome. Nat Rev Dis Primer 5:18
Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML (1992) Tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene 7:1471–1480
Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML (1994) Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 8:1897–1909
Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD (2004) Angiopoietin1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1. Genes Dev 18:1060–1071
Fachinger G, Deutsch U, Risau W (1999) Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor tie-2. Oncogene 18:5948–5953
Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP (2007) Angiopoietin-1 requires P190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem 282:23910–23918
Ziegler T, Horstotte J, Schwab C (2013) Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest 123:3436–3445
Ghosh CC, David S, Zhang R et al (2016) Gene control of tyrosine kinase TIE2 and vascular manifestations of infections. Proc Natl Acad Sci USA 113:2472–2477. https://doi.org/10.1073/pnas.1519467113
Thamm K, Schrimpf C, Retzlaff J, Idowu TO, Meurs M, Zijlstra JG, Ghosh CC, Zeitvogel J, Werfel TA, Haller H, Parikh SM, David S (2018) Molecular regulation of acute Tie2 suppression in sepsis. Crit Care Med 46:928–936
Idowu TO, Etzrodt V, Seeliger B et al (2020) Identification of specific Tie2 cleavage sites and therapeutic modulation in experimental sepsis. Elife 9:e59520. https://doi.org/10.7554/eLife.59520
Koh GY (2013) Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med 19:31–39
Leligdowicz A, Melissa R-G, Wright J, Crowley VM, Kain KC (2018) Endothelial activation: the Ang/Tie axis in sepsis. Front Immunol 9:838
Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel–Palade bodies. Blood 103:4150–4156
Mandriota SJ, Pepper MS (1998) Regulation of angiopoietin-2 MRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 83:852–859
Korhonen EA, Lampinen A, Giri H, Anisimov A, Kim M, Allen B, Fang S, D’Amico G, Sipilä TJ, Lohela M (2016) Tie1 controls angiopoietin function in vascular remodeling and inflammation. J Clin Investig 126:3495–3510
David S, Mukerjee A, Ghosh CC, Yano M, Khankin EV, Wenger JB, Karumanchi SA, Shapiro NI, Parikh SM (2012) Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis*. Crit Care Med 40:3034–3041
Parikh SM (2017) The angiopoietin-Tie2 signaling axis in systemic inflammation. J Am Soc Nephrol 28:1973
Gavrilovskaya IN, Gorbunova EE, Mackow NA, Mackow ER (2008) Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit Hantavirus directed permeability. J Virol 82:5797–5806
Vuong NL, Lam PK, Ming DKY, Duyen HTL, Nguyen NM, Tam DTH, Duong THK, Chau NV, Chanpheaktra N, Lum LCS (2021) Combination of inflammatory and vascular markers in the febrile phase of dengue is associated with more severe outcomes. Elife 10:67460
Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, Krudsood S, Looareesuwan S, John CC, Liles WC (2009) Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS ONE 4:4912
Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR, Mitchell D, Wickersham N, Bernard GR, Matthay MA (2010) Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma Int Infect Crit Care 68:1121–1127
Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, Fuchs BD, Lanken PN, Albelda SM, Rushefski M (2011) ANGPT2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. Am J Respir Crit Care Med 183:1344–1353
Heijden M, Nieu Amerongen GP, Hinsbergh VWM, Groeneveld AJ (2010) The interaction of soluble TIE2 with angiopoietins and pulmonary vascular permeability in septic and nonseptic critically ill patients. Shock 33:263–268
Gopal S (2020) Syndecans in inflammation at a glance. Front Immunol 11:227. https://doi.org/10.3389/fimmu.2020.00227
**an X, Gopal S, Couchman JR (2010) Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res 339:31–46. https://doi.org/10.1007/s00441-009-0829-3
Götte M (2003) Syndecans in inflammation. FASEB J 17:575–591. https://doi.org/10.1096/fj.02-0739rev
Halden Y, Rek A, Atzenhofer W et al (2004) Interleukin-8 binds to syndecan-2 on human endothelial cells. Biochem J 377:533–538. https://doi.org/10.1042/bj20030729
Choi S, Chung H, Hong H et al (2017) Inflammatory hypoxia induces syndecan-2 expression through IL-1b-mediated FOXO3a activation in colonic epithelia. FASEB J 31:1516–1530. https://doi.org/10.1096/fj.201601098R
Götte M, Joussen AM, Klein C et al (2002) Role of syndecan-1 in leukocyte–endothelial interactions in the ocular vasculature. Invest Ophthalmol Vis Sci 43:1135–1141
Voyvodic PL, Min D, Liu R et al (2014) Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem 289:9547–9559
Echtermeyer F, Streit M, Wilcox-Adelman S et al (2001) Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Investig 107:R9–R14
Hyun Y-M, Lefort CT, Kim M (2009) Leukocyte integrins and their ligand interactions. Immunol Res 45:195–208. https://doi.org/10.1007/s12026-009-8101-1
Arokiasamy S, Balderstone MJM, De Rossi G, Whiteford JR (2020) Syndecan-3 in inflammation and angiogenesis. Front Immunol 10:3031. https://doi.org/10.3389/fimmu.2019.03031
Gopal S, Arokiasamy S, Pataki C et al (2021) Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol 11:200377. https://doi.org/10.1098/rsob.200377
Tinholt M, Stavik B, Louch W et al (2015) Syndecan-3 and TFPI colocalize on the surface of endothelial-, smooth muscle-, and cancer cells. PLoS ONE 10:e0117404. https://doi.org/10.1371/journal.pone.0117404
Vuong TT, Reine TM, Sudworth A et al (2015) Syndecan-4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing. J Histochem Cytochem 63:280–292. https://doi.org/10.1369/0022155415568995
Jung C, Fuernau G, Muench P et al (2015) Impairment of the endothelial glycocalyx in cardiogenic shock and its prognostic relevance. Shock 43:450–455. https://doi.org/10.1097/SHK.0000000000000329
Sullivan RC, Rockstrom MD, Schmidt EP, Hippensteel JA (2021) Endothelial glycocalyx degradation during sepsis: causes and consequences. Matrix Biol Plus. 12:100094
Schmidt EP, Li G, Li L, Fu Li, Yang Y, Overdier KH, Douglas IS, Linhardt RJ (2014) The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem 289:8194–8202
Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F (2019) Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care 23:259
Fisher J, Linder A, Bentzer P (2019) Elevated plasma glypicans are associated with organ failure in patients with infection. Intens Care Med Exp 7:2
Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM (2012) The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 18:1217–1223
Wang L, Huang X, Kong G, Xu H, Li J, Hao D, Wang T, Han S, Han C, Sun Y, Liu X, Wang X (2016) Ulinastatin attenuates pulmonary endothelial glycocalyx damage and inhibits endothelial heparanase activity in LPS-induced ARDS. Biochem Biophys Res Commun 478:669–675
Martin L, Santis R, Koczera P, Simons N, Haase H, Heinbockel L, Brandenburg K, Marx G, Schuerholz T, Stover CM (2015) The synthetic antimicrobial peptide 19–2.5 interacts with heparanase and heparan sulfate in murine and human sepsis. PLoS ONE 10:0143583
Lygizos MI, Yang Y, Altmann CJ, Okamura K, Hernando AA, Perez MJ, Smith LP, Koyanagi DE, Gandjeva A, Bhargava R, Tuder RM, Faubel S, Schmidt EP (2013) Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep 1:00153. https://doi.org/10.1002/phy2.153
Vreys V, David G (2007) Mammalian heparanase: what is the message? J Cell Mol Med 11:427–452
Pape T, Hunkemöller AM, Kümpers P et al (2021) Targeting the “sweet spot” in septic shock—a perspective on the endothelial glycocalyx regulating proteins Heparanase-1 and -2. Matrix Biol Plus 12:100095. https://doi.org/10.1016/j.mbplus.2021.100095
Stahl K, Hillebrand UC, Kiyan Y et al (2021) Effects of therapeutic plasma exchange on the endothelial glycocalyx in septic shock. Intensive Care Med Exp 9:57. https://doi.org/10.1186/s40635-021-00417-4
Jung H (2020) Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg 47:297–300
Fraser JRE, Laurent TC, Laurent U (1997) Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242:27–33
Broekhuizen LN, Mooij HL, Kastelein JJP, Stroes ESG, Vink H, Nieuwdorp M (2009) Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr Opin Lipidol 20:57–62
Yagmur E, Koch A, Haumann M, Kramann R, Trautwein C, Tacke F (2012) Hyaluronan serum concentrations are elevated in critically ill patients and associated with disease severity. Clin Biochem 45:82–87
Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, Ammons LA, Hiller TD, Suflita MA, Yu Y, Chen Y, Zhang F, Cothren Burlew C, Edelstein CL, Douglas IS, Linhardt RJ (2016) Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med 194:439–449. https://doi.org/10.1164/rccm.20151
Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L (2011) Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. https://doi.org/10.1126/scisignal.2001868
Bode W, Franz-Xaver G-R, Stockler W (1993) Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins.’ FEBS Lett 331:134–140
Pugin J, Widmer MC, Kossodo S, Liang CM, Preas HL, Suffredini AF (1999) Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol 20:458–464
Nakamura T (1998) Modulation of plasma metalloproteinase-9 concentrations and peripheral blood monocyte mRNA levels in patients with septic shock: effect of fiber-immobilized polymyxin B treatment. Am J Med Sci 316:355–360
Yang X (2018) A disintegrin and metalloproteinase 15 mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc Res 114:1752–1763
Dreymueller D, Martin C, Kogel T, Pruessmeyer J, Hess FM, Horiuchi K, Uhlig S, Ludwig A (2012) Lung endothelial ADAM17 regulates the acute inflammatory response to lipopolysaccharide. EMBO Mol Med 4:412–423
Pruessmeyer J, Martin C, Hess FM, Schwarz N, Schmidt S, Kogel T, Hoettecke N, Schmidt B, Sechi A, Uhlig S, Ludwig A (2010) A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem 285:555–564
Inkinen N, Pettila V, Lakkisto P, Kuitunen A, Jukarainen S, Bendel S, Inkinen O, Ala-Kokko T, Vaara ST (2019) Association of endothelial and glycocalyx injury biomarkers with fluid administration, development of acute kidney injury, and 90-day mortality: data from the FINNAKI observational study. Ann Intensive Care. https://doi.org/10.1186/s13613019-0575-y
Lukasz A, Kumpers P, David S (2012) Role of angiopoietin/tie2 in critical illness: promising biomarker, disease mediator, and therapeutic target? Sci 2012:1–8
Lukasz A, Hillgruber C, Oberleithner H, Kusche-Vihrog K, Pavenstädt H, Rovas A, Hesse B, Goerge T, Kümpers P (2017) Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc Res 113:671–680
Nam EJ, Park PW (2012) Shedding of cell membrane-bound proteoglycans. Methods Mol Biol 836:291–305
Becker BF, Jacob M, Leipert S, Salmon AHJ, Chappell D (2015) Degradation of the endothelial glycocalyx in clinical settings: searching for the Sheddases. Br J Clin Pharmacol 80:389–402
Lipowsky HH, Lescanic A (2013) The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc Res 90:80–85
Manon-Jensen T, Multhaupt HAB, Couchman JR (2013) Map** of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS 280:2320
Chen H-R, Chao CH, Liu CC, Ho TS, Tsai HP, Perng GC, Lin YS, Wang JR, Yeh TM, Fernandez-Sesma A (2018) Macrophage migration inhibitory factor is critical for dengue NS1-induced endothelial glycocalyx degradation and hyperpermeability. PLoS Pathog 14:1007033
Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M (2000) Shedding of syndecan-1 and-4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol 148:811–824
Woodcock T (2017) Plasma volume, tissue oedema, and the steady-state Starling principle. BJA Educ 17:74–78. https://doi.org/10.1093/bjaed/mkw035
Wiig H, Rubin K, Reed RK (2003) New and active role of the interstitium in control of interstitial fluid pressure: potential therapeutic consequences. Acta Anaesthesiol Scand Februar 47:111–121
Michel CC (1997) Starling: the formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp Physiol 82:1–30
Little RC, Ginsburg JM (1984) The physiologic basis for clinical edema. Arch Intern Med 144:1661–1664
Michel LJ, Michel CC (2010) Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87:198–210
Reed RK, Rubin K (2010) Transcapillary exchange: role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc Res 87:211–217
Woodcock TE, Woodcock TM (2012) Revised starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth 108:384–394
Bhave GE, Neilson EG (2011) Body fluid dynamics: back to the future. J Am Soc Nephrol 22:2166–2181
Aldecoa C, Llau JV, Nuvials X, Artigas A (2020) Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann Intensive Care 10:85
Miranda M, Balarini M, Caixeta D, Bouskela E (2016) Microcirculatory dysfunction in sepsis: pathophysiology, clinical monitoring, and potential therapies. Am J Physiol Heart Circul Physiol 311:H24–H35. https://doi.org/10.1152/ajpheart.00034.2016
Attuwaybi B, Kozar RA, Gates KS, Moore-Olufemi S, Sato N, Weisbrodt NW (2004) Hypertonic saline prevents inflammation, injury, and impaired intestinal transit after gut ischemia/reperfusion by inducing heme oxygenase 1 enzyme. J Trauma 56:749–758
Balogh Z, Mckinley BA, Cocanour CS, Kozar RA, Valdivia A, Sailors RM (2003) Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg J 138(637–42):642
Galea I (2021) The blood–brain barrier in systemic infection and inflammation. Cell Mol Immunol 18:2489–2501. https://doi.org/10.1038/s41423-021-00757-x
Malbrain MLNG, Malbrain G, Ostermann M (2022) Everything you need to know about deresuscitation. Intensive Care Med 48:1781–1786
Dimopoulou I, Orfanos S, Kotanidou A, Livaditi O, Giamarellos-Bourboulis E, Athanasiou C (2008) Plasma pro- and anti-inflammatory cytokine levels and outcome prediction in unselected critically ill patients. Cytokine 41:263–267
Acheampong A, Vincent JL (2015) A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care Lond Engl 19:251
Samoni S, Vigo V, Bonilla Reséndiz LI, Villa G, Rosa S, Nalesso F (2016) Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit Care 20:95
Boyd J, Forbes J, Nakada T, Walley K, Russell J (2011) Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 39:259–265
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP et al (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575. https://doi.org/10.1056/NEJMoa062200
Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA (2011) Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med 39:2665–2671
Payen D, Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL (2008) A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 12:74
Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL (2009) Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 76:422–427
Heung M, Wolfgram DF, Kommareddi M, Hu Y, Song PX, Ojo AO (2012) Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant 27:956–961
Bellomo R, Cole A, Cole L, Finfer S, Gallagher M, Lee J, Lo S, McArthur C, McGuiness S, Norton R, Myburgh J, Scheinkestel C, Su S (2012) An observational study fluid balance and patient outcomes in the randomized evaluation of normal vs. augmented level of replacement therapy trial. Crit Care Med 40:1753–1760
Barmparas G, Liou D, Lee D, Fierro N, Bloom M, Ley E, Salim A, Bukur M (2014) Impact of positive fluid balance on critically ill surgical patients: a prospective observational study. J Crit Care 29:936–941
Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van RN (2014) Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 46:361–380
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344–353
Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, Laurila JJ, Mildh L, Reinikainen M, Lund V, Parviainen I, Pettila V, Finnaki SG (2012) Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care 16:197
Wang N, Jiang L, Zhu B, Wen Y, ** XM (2015) Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care 19:371
Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA (2020) Fluid overload and mortality in adult critical care patients—a systematic review and meta-analysis of observational studies*. Crit Care Med 48:1862–1870
Aya HD, Ster IC, Fletcher N (2016) Pharmacodynamic analysis of a fluid challenge. Crit Care Med 44:880–891
Nunes TS, Ladeira RT, Bafi AT (2014) Duration of hemodynamic effects of crystalloids in patients with circulatory shock after initial resuscitation. Ann Intensive Care 4:25
Lankadeva YR, Kosaka J, Iguchi N (2019) Effects of fluid bolus therapy on renal perfusion, oxygenation, and function in early experimental septic kidney injury. Crit Care Med 47:e36–e43
Sánchez M, Jimenez-Lendinez M, Cidoncha M (2011) Comparison of fluid compartments and fluid responsiveness in septic and non-septic patients. Anaesth Intensive Care 39:1022–1029
Dull RO, Hahn RG (2023) Hypovolemia with peripheral edema: what is wrong? Crit Care 27:206. https://doi.org/10.1186/s13054-023-04496-5
Delaney AP, Dan A, McCaffrey J, Finfer S (2011) The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 39:386–391
Connolly CM, Kramer GC, Hahn RG, Chaisson NF, Svensén C, Kirschner RA, Hastings DA, Chinkes DL, Prough DS (2003) Isoflurane but not mechanical ventilation promotes extravascular fluid accumulation during crystalloid volume loading. Anesthesiology 98:670–681
Hahn RG, Lyons G (2016) The half-life of infusion fluids: an educational review. Eur J Anaesthesiol 33:475–482
Harrois A, Duranteau J (2016) Pathophysiology of severe capillary leak. Oxford University Press
Zhang W, Gu Y, Zhao Y et al (2023) Focused liquid ultrasonography in dropsy protocol for quantitative assessment of subcutaneous edema. Crit Care 27:114. https://doi.org/10.1186/s13054-023-04403-y
Brodovicz KG, McNaughton K, Uemura N et al (2009) Reliability and feasibility of methods to quantitatively assess peripheral edema. Clin Med Res 7:21–31. https://doi.org/10.3121/cmr.2009.819
Lee YH, Lee JD, Kang DR, Hong J, Lee JM (2017) Bioelectrical impedance analysis values as markers to predict severity in critically ill patients. J Crit Care 40:103–107
Chung YJ, Kim EY (2021) Usefulness of bioelectrical impedance analysis and ECW ratio as a guidance for fluid management in critically ill patients after operation. Sci Rep 11:12168
Yilmaz Z (2014) Evaluation of fluid status related parameters in hemodialysis and peritoneal dialysis patients: clinical usefulness of bioimpedance analysis. Medicina (Kaunas) 50:269–274
Buffa R, Mereu E, Comandini O, Ibanez ME, Marini E (2014) Bioelectrical impedance vector analysis (BIVA) for the assessment of two-compartment body composition. Eur J Clin Nutr 68:1234–1240
Barbosa-Silva MCG, Barros AJD (2005) Bioelectric impedance and individual characteristics as prognostic factors for postoperative complications. Clin Nutr 24:830–838
Middel C, Stetzuhn M, Sander N et al (2023) Perioperative advanced haemodynamic monitoring of patients undergoing multivisceral debulking surgery: an observational pilot study. Intensive Care Med Exp 11:61. https://doi.org/10.1186/s40635-023-00543-1
Chandra J, Hoz MA, Lee G, Lee A, Thoral P, Elbers P (2022) A novel vascular leak index identifies sepsis patients with a higher risk for in-hospital death and fluid accumulation. Crit Care 26:103
Feldheiser A, Gelman S, Chew M, Stopfkuchen-Evans M (2021) Vasopressor effects on venous return in septic patients: a review. Eur J Anaesthesiol 38:659–663. https://doi.org/10.1097/EJA.0000000000001508
Gavelli F, Shi R, Teboul J-L et al (2022) Extravascular lung water levels are associated with mortality: a systematic review and meta-analysis. Crit Care 26:202. https://doi.org/10.1186/s13054-022-04061-6
Monnet X, Teboul JL (2017) Transpulmonary thermodilution: advantages and limits. Crit Care 21:147
Tagami T, Kushimoto S, Yamamoto Y, Atsumi T, Tosa R, Matsuda K (2010) Validation of extravascular lung water measurement by single transpulmonary thermodilution: human autopsy study. Crit Care 14:162
Dres M, Teboul JL, Anguel N, Guerin L, Richard C, Monnet X (2014) Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema. Crit Care Med 42:1882–1889
Kushimoto S, Taira Y, Kitazawa Y et al (2012) The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: a prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome. Crit Care 16:R232. https://doi.org/10.1186/cc11898
Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C (2007) Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express 15:15101–15114. https://doi.org/10.1364/oe.15.01510
Bruno RR, Hernandez G, Wollborn J et al (2023) Microcirculation information in clinical decision making: Rome wasn’t built in a day. Intensive Care Med. https://doi.org/10.1007/s00134-023-07216-3
Tanaka S, Harrois A, Nicolai C, Flores M, Hamada S, Vicaut E, Duranteau J (2015) Qualitative real-time analysis by nurses of sublingual microcirculation in intensive care unit: the MICRONURSE study. Crit Care 19:388
Naumann DN, Mellis C, Husheer SL, Hopkins P, Bishop J, Midwinter MJ, Hutchings SD (2016) Real-time point of care microcirculatory assessment of shock: design, rationale and application of the point of care microcirculation (POEM) tool. Crit Care 20:310. https://doi.org/10.1186/s1305
Bruno RR, Wollborn J, Fengler K et al (2023) Direct assessment of microcirculation in shock: a randomized-controlled multicenter study. Intensive Care Med 49:645–655. https://doi.org/10.1007/s00134-023-07098-5
Lee DH, Dane MJ, Berg BM, Boels MG, Teeffelen JW, Mutsert R, Heijer M, Rosendaal FR, Vlag J, Zonneveld AJ (2014) Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE 9:e96477
Ince C, Boerma EC, Cecconi M, Backer D, Shapiro NI, Duranteau J, Pinsky MR, Artigas A, Teboul JL, Reiss IKM (2018) Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med 44:281–299
De Backer D, Creteur J, Preiser J-C et al (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 166:98–104. https://doi.org/10.1164/rccm.200109-016oc
Rovas A, Seidel LM, Vink H et al (2019) Association of sublingual microcirculation parameters and endothelial glycocalyx dimensions in resuscitated sepsis. Crit Care 23:260. https://doi.org/10.1186/s13054-019-2542-2
Malbrain ML, Mythen MG, Rice TW, Wuyts S (2018) It is time for improved fluid stewardship. ICU Manage Pract 18:158–162
Ushiyama A, Kataoka H, Iijima T (2016) Glycocalyx and its involvement in clinical pathophysiologies. J Intensive Care 4:59. https://doi.org/10.1186/s4056
Alphonsus CS, Rodseth RN (2014) The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 69:777–784
Jacob M, Bruegger D, Rehm M (2007) The endothelial glycocalyx affords compatibility of Starling’s principle and high cardiac interstitial albumin levels. Cardiovasc Res 73:575–586
Adamson RH, Clark JF, Radeva M, Kheirolomoom A, Ferrara KW, Curry FE (2014) Albumin modulates S1P delivery from red blood cells in perfused microvessels: mechanism of the protein effect. Am J Physiol Heart Circ Physiol 306:1011–1017. https://doi.org/10.1152/ajpheart.00829.2013
Torres GE, Gainetdinov RR, Caron MG (2003) Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci 4:13–25. https://doi.org/10.1016/s0735-1097(02)02168-x
Kremer H, Celine B-M, Tesse A, Gallois Y, Mercat A, Henrion D, Andriantsitohaina R, Asfar P, Meziani F (2011) Human serum albumin improves endothelial dysfunction and survival during experimental endotoxemia: concentration-dependent properties. Crit Care Med 39:1414–1422. https://doi.org/10.1097/CCM.0b013
Lang JDJ, Figueroa M, Chumley P (2004) Albumin and hydro- xyethyl starch modulate oxidative inflammatory injury to vascular endothelium. Anesthesiology 100:51–58
Tatara T (2003) The contribution of solute-solvent exchange at the membrane surface to the reduction by albumin of the hydraulic permeability coefficient of an artificial semipermeable membrane. Anesth Analg 97:1137–1142
Cosenza L, Donatti A, Cecero E, Carlucci M, Adembri C, Fusi F (2012) Effects of albumin infusion on LPS-induced damage of mesenteric microcirculation: 12AP1-9. Eur J Anaesthesiol 29:176–177
Rehm M, Zahler S, Lötsch M, Welsch U, Conzen P, Jacob M, Becker BF (2004) Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology 100:1211–1223. https://doi.org/10.1097/00000
Milford EM, Reade MC (2019) Resuscitation fluid choices to preserve the endothelial glycocalyx. Crit Care 23:77. https://doi.org/10.1186/s1305
Torres LN, Chung KK, Salgado CL, Dubick MA, Torres Filho IP (2017) Low volume resuscitation with normal saline is associated with microvascular endothelial dysfunction after hemorrhage in rats, compared to colloids and balanced crystalloids. Crit Care 21:160. https://doi.org/10.1186/s1305
Hariri G, Joffre J, Maury E et al (2022) Ten myths about albumin: do not forget the endothelium. Intensive Care Med 48:1097–1098. https://doi.org/10.1007/s00134-022-06740-y
Sakr Y, Bauer M, Nierhaus A et al (2020) Randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS): protocol for a randomized controlled trial. Trials 21:1002. https://doi.org/10.1186/s13063-020-04921-y
Raoufinia R, Mota A, Keyhanvar N et al (2016) Overview of albumin and its purification methods. Adv Pharm Bull 6:495–507. https://doi.org/10.15171/apb.2016.063
Zdolsek M, Wuethrich PY, Gunnström M, Zdolsek JH, Hasselgren E, Beilstein CM (2022) Plasma disappearance rate of albumin when infused as a 20% solution. Crit Care 26:104
Zdolsek M, Hahn RG (2022) Kinetics of 5% and 20% albumin: a controlled crossover trial in volunteers. Acta Anaesthesiol Scand 66:847–858
Hahn RG, Zdolsek M, Hasselgren E, Zdolsek J, Björne H (2019) Fluid volume kinetics of 20% albumin. Br J Clin Pharmacol Juni 85:1303–1311
Yanase F, Tosif SH, Churilov L et al (2021) A randomized, multicenter, open-label, blinded end point, phase 2, feasibility, efficacy, and safety trial of preoperative microvascular protection in patients undergoing major abdominal surgery. Anesth Analg 133:1036–1047. https://doi.org/10.1213/ANE.0000000000005667
Caironi P, Tognoni G, Masson S et al (2014) Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 370:1412–1421. https://doi.org/10.1056/NEJMoa1305727
Pesonen E, Vlasov H, Suojaranta R, Hiippala S, Schramko A, Wilkman E (2022) Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA 328:251
Lee TH, Kuo G, Chang CH, Huang YT, Yen CL, Lee CC (2021) Diuretic effect of co-administration of furosemide and albumin in comparison to furosemide therapy alone: an updated systematic review and meta-analysis. PLoS ONE 1:0260312
Wiig H, Swartz MA (2012) Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 92:1005–1060. https://doi.org/10.1152/physrev.00037.2011
Földi E, Sauerwald A, Hennig B (2000) Effect of complex decongestive physiotherapy on gene expression for the inflammatory response in peripheral lymphedema. Lymphology 33:151–157
Bozkurt M, Palmer LJ, Guo Y (2017) Effectiveness of decongestive lymphatic therapy in patients with lymphedema resulting from breast cancer treatment regardless of previous lymphedema treatment. Breast J März 23:154–158
Lasinski BB, McKillip Thrift K, Squire D et al (2012) A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R 4:580–601. https://doi.org/10.1016/j.pmrj.2012.05.003
Brix B, Apich G, Roessler A et al (2020) Fluid shifts induced by physical therapy in lower limb lymphedema patients. JCM 9:3678. https://doi.org/10.3390/jcm9113678
Schick MA, Wunder C, Wollborn J et al (2012) Phosphodiesterase-4 inhibition as a therapeutic approach to treat capillary leakage in systemic inflammation. J Physiol 590:2693–2708. https://doi.org/10.1113/jphysiol.2012.232116
Wollborn J, Siemering S, Steiger C et al (2019) Phosphodiesterase-4 inhibition reduces ECLS-induced vascular permeability and improves microcirculation in a rodent model of extracorporeal resuscitation. Am J Physiol Heart Circul Physiol 316:H751–H761. https://doi.org/10.1152/ajpheart.00673.2018
Schlegel N, Baumer Y, Drenckhahn D, Waschke J (2009) Lipopolysaccharide-induced endothelial barrier breakdown is cyclic adenosine monophosphate dependent in vivo and in vitro*. Crit Care Medicine 37:1735–1743. https://doi.org/10.1097/CCM.0b013e31819deb6a
Spindler V, Schlegel N, Waschke J (2010) Role of GTPases in control of microvascular permeability. Cardiovasc Res 87:243–253. https://doi.org/10.1093/cvr/cvq086
Schmidt EP, Damarla M, Rentsendorj O et al (2008) Soluble guanylyl cyclase contributes to ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 295:L1056-1065. https://doi.org/10.1152/ajplung.90329.2008
Gonçalves RL, Lugnier C, Keravis T et al (2009) The flavonoid dioclein is a selective inhibitor of cyclic nucleotide phosphodiesterase type 1 (PDE1) and a cGMP-dependent protein kinase (PKG) vasorelaxant in human vascular tissue. Eur J Pharmacol 620:78–83
Chan S, Yan C (2011) PDE1 isozymes, key regulators of pathological vascular remodeling. Curr Opin Pharmacol 11:720–724
Nagel DJ, Aizawa T, Jeon K-I et al (2006) Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res 98:777–784
Murray F, Patel HH, Suda RY et al (2007) Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol 292:294–303
Schermuly RT, Pullamsetti SS, Kwapiszewska G et al (2007) Phosphodiesterase 1 upregulation in pulmonary arterial hypertension target for reverse-remodeling therapy. Circulation 115:2331–2339
Vemulapalli S, Watkins RW, Chintala M et al (1996) Antiplatelet and antiproliferative effects of SCH 51866, a novel type 1 and type 5 phosphodiesterase inhibitor. J Cardiovasc Pharmacol 28:862–869
Seybold J, Thomas D, Witzenrath M et al (2005) Tumor necrosis factor a-dependent expression of phosphodiesterase 2: role in endothelial hyperpermeability. Blood 105:3569–3576
Dunkern TR, Hatzelmann A (2005) The effect of Sildenafil on human platelet secretory function is controlled by a complex interplay between phosphodiesterases 2, 3 and 5. Cell Signal 17:331–339
Bubb KJ, Trinder SL, Baliga RS et al (2014) Inhibition of phosphodiesterase 2 augments cGMP and cAMP signaling to ameliorate pulmonary hypertension. Circulation 130:496
Nikpour M, Sadeghian H, Saberi MR et al (2010) Design, synthesis and biological evaluation of 6(benzyloxy)-4-methylquinolin-2 (1H)-one derivatives as PDE3 inhibitors. Bioorg Med Chem 18:855–862
Shakur Y, Holst LS, Landstrom TR et al (2000) Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog Nucleic Acid Res Mol Biol 66:241–277
Aizawa T, Wei H, Miano JM et al (2003) Role of phosphodiesterase 3 in NO/cGMP-mediated antiinflammatory effects in vascular smooth muscle cells. Circ Res 93:406–413
Rotella DP (2002) Phosphodiesterase 5 inhibitors: current status and potential applications. Nat Rev Drug Discov 1:674–682
Kass DA, Champion HC, Beavo JA (2007) Phosphodiesterase type 5 expanding roles in cardiovascular regulation. Circ Res 101:1084–1095
Giannetta E, Feola T, Gianfrilli D et al (2014) Is chronic inhibition of phosphodiesterase type 5 cardioprotective and safe? A meta-analysis of randomized controlled trials. BMC Med 12:1
Cristina RT, Dehelean C, Dumitrescu E et al (2010) Structural basis of phosphodiesterase 6 inhibition by the C-terminal region of the g-subunit. EMBO J 43:3613–3622
Yang G, McIntyre KW, Townsend RM et al (2003) Phosphodiesterase 7A-deficient mice have functional T cells. J Immunol 171:6414–6420
Redondo M, Brea J, Perez DI et al (2012) Effect of phosphodiesterase 7 (PDE7) inhibitors in experimental autoimmune encephalomyelitis mice. Discovery of a new chemically diverse family of compounds. J Med Chem 55:3274–3284
Wang P, Wu P, Egan RW, Billah MM (2001) Human phosphodiesterase 8A splice variants: cloning, gene organization, and tissue distribution. Gene 280:183–194
Fisher DA, Smith JF, Pillar JS et al (1998) Identification and distribution of different mRNA variants produced by differential splicing in the human phosphodiesterase 9A gene. Biochem Biol Chem 273:15559–15564
Lee DI, Zhu G, Sasaki T et al (2015) Phosphodiesterase 9A controls nitric-oxide independent cGMP and hypertrophic heart disease. Nature 519:472–476
Strick C, Schmidt C, Menniti F (2006) PDE10A: a striatum-enriched, dual-substrate phosphodiesterase, cyclic nucleotide phosphodiesterases in health and disease. CRC Press, USA
Makhlouf A, Kshirsagar A, Niederberger C et al (2006) Phosphodiesterase 11: a brief review of structure, expression and function. Int J Impot Res 18:501–509
Su JB (2015) Vascular endothelial dysfunction and pharmacological treatment. World J Cardiol 7:719–741. https://doi.org/10.4330/wjc.v7.i11.719
Fulton D, Gratton JP, McCabe TJ et al (1999) Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601
Chen CA, Wang TY, Varadharaj S et al (2010) S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468:1115–1118
Saravi B, Li Z, Lang CN et al (2021) The tissue renin–angiotensin system and its role in the pathogenesis of major human diseases: Quo Vadis? Cells 10:650. https://doi.org/10.3390/cells10030650
Félétou M, Vanhoutte PM (2009) EDHF:an update. Clin Sci 117:139–155. https://doi.org/10.1007/s00424-010-0817-1
Fleming I, Michaelis UR, Bredenkötter D et al (2001) Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9)is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res 88:44–51. https://doi.org/10.1093/cvr/cvu015
Blanco-Rivero J, Cachofeiro V, Lahera V et al (2005) Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension 83:21. https://doi.org/10.1161/01.HYP.0000171479.36880.17
David S, Russell L, Castro P et al (2023) Research priorities for therapeutic plasma exchange in critically ill patients. Intensive Care Med Exp 11:26. https://doi.org/10.1186/s40635-023-00510-w
David S, Bode C, Putensen C et al (2021) Adjuvant therapeutic plasma exchange in septic shock. Intensive Care Med 47:352–354. https://doi.org/10.1007/s00134-020-06339-1
Stahl K, Schmidt JJ, Seeliger B et al (2020) Effect of therapeutic plasma exchange on endothelial activation and coagulation-related parameters in septic shock. Crit Care 24:71. https://doi.org/10.1186/s13054-020-2799-5
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by departmental funds.
Author information
Authors and Affiliations
Contributions
Draft manuscript preparation: B.S., U.G., L.O.H., C.J., S.D., A.F., M.S.E., J.W. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saravi, B., Goebel, U., Hassenzahl, L.O. et al. Capillary leak and endothelial permeability in critically ill patients: a current overview. ICMx 11, 96 (2023). https://doi.org/10.1186/s40635-023-00582-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-023-00582-8