Abstract
Tau neurofibrillary tangle (NFT) pathology in the medial temporal lobe (MTL) is closely linked to neurodegeneration, and is the early pathological change associated with Alzheimer’s disease (AD). To elucidate patterns of structural change in the MTL specifically associated with tau pathology, we compared high-resolution ex vivo MRI scans of human postmortem MTL specimens with histology-based pathological assessments of the MTL. MTL specimens were obtained from twenty-nine brain donors, including patients with AD, other dementias, and individuals with no known history of neurological disease. Ex vivo MRI scans were combined using a customized groupwise diffeomorphic registration approach to construct a 3D probabilistic atlas that captures the anatomical variability of the MTL. Using serial histology imaging in eleven specimens, we labelled the MTL subregions in the atlas based on cytoarchitecture. Leveraging the atlas and neuropathological ratings of tau and TAR DNA-binding protein 43 (TDP-43) pathology severity, morphometric analysis was performed to correlate regional MTL thickness with the severity of tau pathology, after correcting for age and TDP-43 pathology. We found significant correlations between tau pathology and thickness in the entorhinal cortex (ERC) and stratum radiatum lacunosum moleculare (SRLM). When focusing on cases with low levels of TDP-43 pathology, we found strong associations between tau pathology and thickness in the ERC, SRLM and the subiculum/cornu ammonis 1 (CA1) subfields of the hippocampus, consistent with early Braak stages.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) pathology is characterized by phosphorylated-tau in the form of neurofibrillary tangles (NFTs) and extra-cellular deposits of amyloid-beta (Aβ) [1,2,3], which are thought to lead to neurodegeneration and cognitive decline. Measures of Aβ, tau and neurodegeneration (A/T/N) can be obtained using positron emission tomography (PET), cerebrospinal fluid (CSF) or structural magnetic resonance imaging (MRI), and are now being used to biologically define AD within a research framework [4]. Compared to Aβ, the accumulation of NFTs in particular is strongly correlated with neurodegeneration and cognitive decline [5,9, 10]. Eventually, the NFTs spread to other cortical regions of the brain. Because of the MTL’s early involvement, its subregions are expected to be among the most sensitive brain regions to early pathological changes in AD.
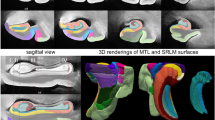
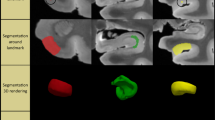
A growing number of studies have used in vivo structural MRI to derive measurements of subtle volumetric changes in the MTL caused by neurodegeneration [11, 24]. Most recently, Wisse et al. conducted a postmortem study which investigated the association between the thickness of MTL subregions in ex vivo MRI and semi-quantitative ratings of different neurodegenerative pathologies derived from histology samples taken from the contralateral hemisphere [ Brain hemispheres were obtained from twenty-nine donors; one specimen from the brain bank operated by the National Disease Research Interchange (NDRI), sixteen specimens from autopsies performed at the University of Pennsylvania Center for Neurodegenerative Disease Research (CNDR) and twelve specimens from the University of Castilla-La Mancha (UCLM) Human Neuroanatomy Laboratory (HNL) in Spain. Human brain specimens were obtained in accordance with the University of Pennsylvania Institutional Review Board guidelines, and the Ethical Committee of UCLM. Where possible, pre-consent during life and, in all cases, next-of-kin consent at death was given. CNDR hemispheres were fixed in 10% formalin solution for at least 30 days before extracting intact MTL blocks. HNL cases were fixed by perfusion with 4% paraformaldehyde through both carotid arteries. The blocks were then imaged on a Varian 9.4 T animal scanner at a 200 × 200 × 200 µm3 resolution. Details of the imaging protocol are provided in Additional file 1: Section 1.1 and Additional file 1: Fig. S7. Following MRI scanning, each of the specimens underwent histological processing for neuropathological examination and neuroanatomical analysis. In twenty-seven specimens, dense serial histology was performed. The specimens were cut into 2 cm blocks using custom molds that were 3D printed to fit each MTL specimen, cryoprotected and sectioned at 50 μm intervals in a sliding microtome coupled to a freezing unit (Microm, Heidelberg). For neuropathological diagnosis, we were interested in examining the anterior ERC at the mid-level of the amygdala, and the dentate gyrus (DG) and CA subfields at the mid-level of the body of the hippocampus. Therefore, for the remaining two specimens that did not undergo serial histology, two tissue blocks were cut at the level of the amygdala and hippocampal body, cryoprotected and sectioned into 50 μm sections. For each of the specimens, two adjacent sections were sampled at the mid-level of the amygdala and the hippocampal body for immunohistochemistry. In each case, the two sections were immunostained for tau and TDP-43 using anti-human PHF-Tau (monoclonal antibody (mAb), mouse, Thermo Scientific, Product Number MN1020, 1:500) and 66318-1-Ig Anti-phospho (409/410) TDP-43 (mAb, mouse, Proteintech, 1:350) respectively. Sections were then mounted on 7.5 cm × 5 cm slides and digitally scanned at 20× resolution. Regional thickness analysis was performed using histopathology ratings of tau and TDP-43 pathologies derived from the MTL both ipsilateral and contralateral to the one that was scanned. Semi-quantitative ratings for tau and TDP-43 pathology were obtained for the contralateral hemisphere by obtaining tissue samples at the time of autopsy and are available for a subset of twenty-eight donors (CNDR and HNL specimens) in three MTL regions routinely examined in the CNDR neuropathology evaluations [29]. For each of the specimens, the boundaries between MTL subregions cornu ammonis (CA) 1, CA2, CA3, DG, SUB, presubiculum, parasubiculum, hippocampal amygdala transition area (HATA), SRLM, ERC, BA35, BA36, area TE and the parahippocampal cortex (areas TF and TH) were identified on the basis of cytoarchitectural features in the Nissl stained sections following the anatomical rules presented in the Atlas of the Human Brain by Mai et al. [30]. Annotations were performed on each histology slice by the team of neuroanatomists at UCLM (the hippocampal subfields and ERC were annotated by M.M.A, E.A.P, M.M.R, M.M.L, C.R.P, S.C.S, J.C.D, M.C.P and F.M.R., supervised by R.In; R.In annotated the boundaries of the temporopolar cortex, PRC and PHC, and revised the annotations of the group). The neuroanatomists viewed scanned Nissl slides in an open-source web-based system (https://github.com/pyushkevich/histoannot) and used line drawings and text labels to annotate boundaries between adjacent anatomical regions. Following histology reconstruction and registration to MRI, the boundary annotations were overlaid on the co-registered MRI and histology images. Additionally, the manual segmentations of the whole MTL cortex and SRLM that were used to guide groupwise registration were registered to the histology images to inform the outer MTL boundary. The MRI, histology and MTL segmentation images were displayed side-by-side in ITK-SNAP [31] to facilitate manual tracing of the subfield segmentations in 3D MRI space, which was performed by S.L. with supervision from L.E.M.W and R.In (Additional file 1: Fig. S9). For each of the eleven specimens, the completed segmentations were then mapped to the MRI atlas using the deformable transformations generated by the groupwise registration pipeline. Note that for each specimen, small gaps in the segmentation may exist between blocks. A consensus segmentation of the MRI atlas was obtained by application of voxel-wise majority voting among the eleven segmentations with slight regularization by a Markov Random Field prior. More details on this approach are provided in the supplemental information, Section S1.4.5 of Adler et al. [1: Section 1.4). To test the effects of tau pathology on regional thickness, we fit a general linear model (GLM) at each vertex on both the MTL and SRLM surfaces with the average rating of tau pathology as the independent variable, thickness as the dependent variable, and age and TDP-43 rating as covariates. More details on the statistical analysis are provided in Additional file 1: Section 1.5. The specimens included in this study contain varying neuropathological diagnoses, including AD neuropathologic change and neuropathological diagnoses such as argyrophilic grain disease (AGD), frontotemporal lobar degeneration (FTLD) with TDP-43 inclusions, cerebrovascular disease and Lewy body disease. Figure 1 summarizes the demographic and neuropathology data for this brain donor cohort. The average ipsilateral tau and TDP-43 ratings across the twenty-nine specimens are 1.50 ± 0.97 and 0.54 ± 0.82 respectively. The average age is 74 years (range 44–93 years). Additional file 1: Table S2 provides more detailed demographic data and pathology information for each specimen. Demographic and diagnostic summaries for the twenty-nine brain donors. The tau and TDP-43 pathology ratings refer to the average rating computed from measurements sampled at three medial temporal lobe locations (entorhinal cortex at the mid-level of the amygdala and subiculum/cornu ammonis and dentate gyrus at the mid-level of the hippocampus). Dashed lines are used to indicate the mean value across specimens Figure 2 shows the MRI atlas of the MTL constructed from twenty-nine ex vivo specimens as a synthetic “average” MR image and a consensus MTL subregion segmentation derived from serial histology in eleven specimens. The atlas construction pipeline achieves excellent groupwise alignment between ex vivo MRI scans and captures the average shape of the MTL (Additional file 1: Section 2.1, Additional file 1: Fig. S2, Additional file 1: Fig. S3 and Additional file 1: Fig. S4). Following groupwise registration, each specimen has a pointwise spatial correspondence to this atlas. This correspondence is limited to the region of the MTL cortex which was semi-automatically segmented in each specimen. Figure 3 provides a visualization of the quality of the registration between individual specimen images and the final atlas. In some specimens, the PRC, which includes BA35, was particularly challenging to register due to significant anatomical variability in cortical folding and branching patterns [32, 33]. Overall, the warped specimens look similar to each other following registration, although some minor mis-registrations remain. Quantitative and visual evaluation of atlas quality at different stages of atlas construction, and comparisons with an alternative atlas-building strategy are presented in Additional file 1: Section 2.1. Computational atlas of the medial temporal lobe (MTL) constructed by groupwise registration of the magnetic resonance image (MRI) scans of twenty-nine ex vivo specimens. Three coronal sections are shown ordered from anterior (ant) to posterior (post), indicated as I, II and III, as well as a sagittal and axial section through the MTL. For each section, the “average” MRI is shown with and without the consensus MTL subregion segmentation derived from serial histology in eleven specimens. In the top right, a 3D reconstruction of the MTL atlas is shown along with a 3D brain rendering indicating the location of the MTL within the brain. (med medial, lat lateral, sup superior, inf inferior, SUB subiculum, SRLM stratum radiatum lacunosum moleculare, CA cornu ammonis, DG dentate gyrus, HATA hippocampal amygdala transition area, ERC entorhinal cortex, BA Brodmann Area) Coronal view of the MRI scans of each of the twenty-nine specimens warped into the space of the MRI atlas. The corresponding atlas image is outlined in blue, in the bottom-right corner. The more similar the warped images are, the better the atlas quality. The dashed blue circles point out examples where the perirhinal cortex (region surrounding the collateral sulcus) was particularly challenging to register due to significant variability in cortical folding patterns. The color bar at the bottom of each image indicates the average neurofibrillary tangle (NFT) rating for that specimen. Yellow represents a rating of 0 (no/rare pathology) and red represents a rating of 3 (severe Alzheimer’s disease) Figure 4 shows the results of the pointwise thickness analysis performed on the MTL and SRLM examining the regional effects of tau pathology on cortical thickness using pathology ratings derived from the MTL ipsilateral to the thickness measures. Cases CNDR12 (44 y.o.) and HNL01 (45 y.o.) were excluded from the thickness analyses since these younger cases are outliers in terms of age and including them skews age effects, thereby dampening the associations between pathology and thickness. In this work we only consider the effects of tau and TDP-43 pathology on MTL structure since recent work studying the contribution of mixed pathology to MTL atrophy in AD showed no clear relationship between neurodegeneration and either Aβ or \(\alpha\)-synuclein [34, 9, 29]. These NFT density maps are generated using a weakly supervised deep learning algorithm, trained to specifically detect tangles and pre-tangles on AT8-stained sections and have been shown to correlate strongly with manual NFT counts [29]. Three cases with a primary FTLD-Tau or AGD diagnosis (CNDR01, CNDR06 and CNDR07) were excluded from the analysis since they likely contain 4R-tau inclusions which can be difficult to distinguish from AD-related NFTs. In the remaining twelve cases, we examined the correlation between the average NFT burden computed within each hotspot and the median thickness of each hotspot, and observe very strong correlations, although the hotspot in the ERC does not reach significance (Additional file 1: Fig. S12). These results are weakened when we include age in the model. This is likely due to the small sample size and the fact that age and tau are significantly correlated (average R = 0.61 across the three hotspots). Despite the small dataset, these results are encouraging and suggest that NFTs are indeed playing a role in driving neurodegeneration in the ERC, SUB and SRLM hotspots. As we expand our dataset, collect more quantitative histology data and develop automated methods to extract quantitative measures of the different types of tau and TDP-43 pathology, in future work we will be able to better overcome the above-mentioned limitations and validate the relationship between NFTs and thickness. Overall, our findings provide a more refined understanding of how tau pathology is associated with cortical thinning within the MTL and motivate further characterization of the MTL in AD using detailed ex vivo MRI analysis. The clusters identified from the tau thickness analyses indicate granular MTL regions where in vivo measures of neurodegeneration are expected to be strongly associated with tau pathology. In an exploratory analysis, we attempted to use the ex vivo derived, AD-specific hotspots (Fig. 4B) as biomarkers in a longitudinal analysis of ADNI data (not shown). While the hotspots did not show statistical effects in a group comparison of Aβ negative, tau negative (A−T−) and Aβ positive, tau positive (A+T+) patient groups with mild cognitive impairment, BA35 achieved the strongest group discrimination. The lack of statistically significant group differences with the hotspots may be due to the small ex vivo sample size, clinical heterogeneity in our dataset and difficulties in accurately aligning BA35 in the atlas. Constructing a probabilistic atlas of the MTL has far-reaching applications in AD research beyond the work shown here. Perhaps future analyses leveraging this technology in a patient cohort more consistent with the one we would expect to encounter in an AD clinical trial would result in hotspots that are more sensitive to longitudinal change in the presence of NFT pathology, potentially enabling the development of neurodegeneration biomarkers which are more effective during early AD clinical trials. Furthermore, in future work, 3D quantitative maps of NFT density derived from serial histology imaging will be mapped into ex vivo atlas space to generate a comprehensive probabilistic description of the progression of NFT pathology at each Braak stage [29, 48, 49]. This will allow us to describe NFT topography during the different stages of AD in more detail than current descriptions, which are in 2D and based on selective sampling of the MTL [1, 2]. Additionally, in a future version of the atlas, cytoarchitecture-guided anatomical labels of MTL subregions will be included from a larger number of specimens. Such an atlas would reflect anatomical ground truth and can be used to inform in vivo MRI protocols for segmentation of MTL subregions, thereby improving the accuracy of MRI biomarkers derived from these subregions.Methods
Specimen preparation and MRI
Histological processing and immunohistochemistry
Semi-quantitative neuropathology ratings
Results
Demographics
Computational atlas of the medial temporal lobe
Effects of tau pathology on regional thickness
Availability of data and materials
Anonymized MRI data and intermediate results have been uploaded to https://openneuro.org/ (dataset https://doi.org/10.18112/openneuro.ds003052.v1.1.0). Source code used to construct the atlas and for statistical analysis can be accessed via GitHub (sadhana-r/Penn_exvivoMTLAtlas.git).
References
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259. https://doi.org/10.1007/bf00308809
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278. https://doi.org/10.1016/0197-4580(95)00021-6
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement 14:535–562
Bobinski M, Wegiel J, Tarnawski M, Bobinksi M, Reisberg B, De Leon MJ, Miller DC, Wisniewski HM (1997) Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol 56:414–420. https://doi.org/10.1097/00005072-199704000-00010
Flores R, Wisse LEM, Das SR, **e L, McMillan CT, Trojanowski JQ, Robinson JL, Grossman M, Lee E, Irwin DJ, Yushkevich PA, Wolk DA (2020) Contribution of mixed pathology to medial temporal lobe atrophy in Alzheimer’s disease. Alzheimer’s Dement 16:alz.12079. https://doi.org/10.1002/alz.12079
Von Gunten A, Kövari E, Bussière T, Rivara CB, Gold G, Bouras C, Hof PR, Giannakopoulos P (2006) Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer’s disease. Neurobiol Aging 27:270–277. https://doi.org/10.1016/j.neurobiolaging.2005.02.008
Thaker AA, Weinberg BD, Dillon WP, Hess CP, Cabral HJ, Fleischman DA, Leurgans SE, Bennett DA, Hyman BT, Albert MS, Killiany RJ, Fischl B, Dale AM, Desikan RS (2017) Entorhinal cortex: antemortem cortical thickness and postmortem neurofibrillary tangles and amyloid pathology. Am J Neuroradiol 38:961–965. https://doi.org/10.3174/ajnr.A5133
Braak E, Braak H (1997) Alzheimer’s disease: transiently develo** dendritic changes in pyramidal cells of sector CA1 of the Ammon’s horn. Acta Neuropathol 93:323–325
Thal DR, Holzer M, Rüb U, Waldmann G, Günzel S, Zedlick D, Schober R (2000) Alzheimer-related τ-pathology in the perforant path target zone and in the hippocampal stratum oriens and radiatum correlates with onset and degree of dementia. Exp Neurol 163:98–110. https://doi.org/10.1006/exnr.2000.7380
Olsen RK, Palombo DJ, Rabin JS, Levine B, Ryan JD, Rosenbaum RS (2013) Volumetric analysis of medial temporal lobe subregions in developmental amnesia using high-resolution magnetic resonance imaging. Hippocampus 23:855–860. https://doi.org/10.1002/hipo.22153
Yushkevich PA, Pluta JB, Wang H, **e L, Ding SL, Gertje EC, Mancuso L, Kliot D, Das SR, Wolk DA (2015) Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp 36:258–287. https://doi.org/10.1002/hbm.22627
Robinson JL, Lee EB, **e SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A, Hurtig HI, Siderowf A, Grossman M, McMillan CT, Miller B, Duda JE, Irwin DJ, Wolk D, Elman L, McCluskey L, Chen-Plotkin A, Weintraub D, Arnold SE, Brettschneider J, Lee VM-Y, Trojanowski JQ (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141:2181–2193. https://doi.org/10.1093/brain/awy153
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601
Jack CR, Dickson DW, Parisi JE, Xu YC, Cha RH, O’Brien PC, Edland SD, Smith GE, Boeve BF, Tangalos EG, Kokmen E, Petersen RC (2002) Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 58:750–757. https://doi.org/10.1212/WNL.58.5.750
Kaur B, Himali JJ, Seshadri S, Beiser AS, Au R, McKee AC, Auerbach S, Wolf PA, Decarli CS (2014) Association between neuropathology and brain volume in the Framingham Heart Study. Alzheimer Dis Assoc Disord 28:219–225. https://doi.org/10.1097/WAD.0000000000000032
Whitwell J, Josephs K, Murray M, Neurology KK (2008) MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 71:743–749
Lindberg O, Mårtensson G, Stomrud E, Palmqvist S, Wahlund LO, Westman E, Hansson O (2017) Atrophy of the posterior subiculum is associated with memory impairment, tau- and Aβ pathology in non-demented individuals. Front Aging Neurosci 9:306. https://doi.org/10.3389/fnagi.2017.00306
de Souza LC, Chupin M, Lamari F, Jardel C, Leclercq D, Colliot O, Lehéricy S, Dubois B, Sarazin M (2012) CSF tau markers are correlated with hippocampal volume in Alzheimer’s disease. Neurobiol Aging 33:1253–1257. https://doi.org/10.1016/j.neurobiolaging.2011.02.022
Tardif CL, Devenyi GA, Amaral RSC, Pelleieux S, Poirier J, Rosa-Neto P, Breitner J, Chakravarty MM (2018) Regionally specific changes in the hippocampal circuitry accompany progression of cerebrospinal fluid biomarkers in preclinical Alzheimer’s disease. Hum Brain Mapp 39:971–984. https://doi.org/10.1002/hbm.23897
Das SR, **e L, Wisse LEM, Vergnet N, Ittyerah R, Cui S, Yushkevich PA, Wolk DA (2019) In vivo measures of tau burden are associated with atrophy in early Braak stage medial temporal lobe regions in amyloid-negative individuals. Alzheimer’s Dement 15:1–10. https://doi.org/10.1016/j.jalz.2019.05.009
Marks SM, Lockhart SN, Baker SL, Jagust WJ (2017) Tau and β-amyloid are associated with medial temporal lobe structure, function, and memory encoding in normal aging. J Neurosci 37:3192–3201. https://doi.org/10.1523/JNEUROSCI.3769-16.2017
**e L, Das SR, Wisse LEM, Ittyerah R, Yushkevich PA, Wolk DA (2018) Early tau burden correlates with higher rate of atrophy in transentorhinal cortex. J Alzheimer’s Dis 62:85–92. https://doi.org/10.3233/JAD-170945
Saint-Aubert L, Lemoine L, Chiotis K, Leuzy A, Rodriguez-Vieitez E, Nordberg A (2017) Tau PET imaging: present and future directions. Mol Neurodegener 12:1–21
Wisse L, Ravikumar S, Ittyerah R, Lim SA, Lane J, Bedard ML, **e L, Robinson JL, Schuck T, Grossman M, Lee EB, Tisdall DM, Prabhakaran K, Detre JA, Das SR, Mizsei G, Artacho-Perula E, de Martin MMIO, del Jiménez MMA, Munoz M, Romero FJM, del Rabal MPM, Sánchez SC, González JCD, de la Rosa-Prieto C, Parada MC, Irwin DJ, Trojanowski JQ, Wolk DA, Insausti R, Yushkevich PA (2021) Downstream effects of polypathology on neurodegeneration of medial temporal lobe subregions. Acta Neuropathol Commun 9:128. https://doi.org/10.1186/s40478-021-01225-3
Toledo JB, Van Deerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, **e SX, McBride J, Wood EM, Schuck T, Irwin DJ, Gross RG, Hurtig H, McCluskey L, Elman L, Karlawish J, Schellenberg G, Chen-Plotkin A, Wolk D, Grossman M, Arnold SE, Shaw LM, Lee VMY, Trojanowski JQ (2014) A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimer’s Dement 10:477-484.e1. https://doi.org/10.1016/j.jalz.2013.06.003
Ravikumar S, Wisse L, Gao Y, Gerig G, Yushkevich P (2019) Facilitating manual segmentation of 3D datasets using contour and intensity guided interpolation. In: Proceedings—international symposium on biomedical imaging
Adler DH, Wisse LEM, Ittyerah R, Pluta JB, Ding SL, **e L, Wang J, Kadivar S, Robinson JL, Schuck T, Trojanowski JQ, Grossman M, Detre JA, Elliott MA, Toledo JB, Liu W, Pickup S, Miller MI, Das SR, Wolk DA, Yushkevich PA (2018) Characterizing the human hippocampus in aging and Alzheimer’s disease using a computational atlas derived from ex vivo MRI and histology. Proc Natl Acad Sci U S A 115:4252–4257. https://doi.org/10.1073/pnas.1801093115
Yushkevich PA, López MM, de Martin MMIO, Ittyerah R, Lim S, Ravikumar S, Bedard ML, Pickup S, Liu W, Wang J, Hung LY, Lasserve J, Vergnet N, ** of neurofibrillary tangle burden in the human medial temporal lobe. Brain 139:16–17. https://doi.org/10.1093/BRAIN/AWAB262
Mai JK, Majtanik M, Paxinos G (2015) Atlas of the human brain. Academic Press, London
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Ding S-L, Van Hoesen GW (2010) Borders, extent, and topography of human perirhinal cortex as revealed using multiple modern neuroanatomical and pathological markers. Hum Brain Mapp 31:1359–1379. https://doi.org/10.1002/hbm.20940
Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A (1998) MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol 19:659–671
Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE, Van Deerlin VM, Seeley WW, Miller BL, Lee EB, Lee VMY, Grossman M, Trojanowski JQ (2016) Deep clinical and neuropathological phenoty** of Pick disease. Ann Neurol 79:272–287. https://doi.org/10.1002/ana.24559
Irwin DJ, McMillan CT, **e SX, Rascovsky K, Van Deerlin VM, Coslett HB, Hamilton R, Aguirre GK, Lee EB, Lee VMY, Trojanowski JQ, Grossman M (2018) Asymmetry of post-mortem neuropathology in behavioural-variant frontotemporal dementia. Brain 141:288–301. https://doi.org/10.1093/brain/awx319
**e L, Pluta JB, Das SR, Wisse LEM, Avants BB, Yushkevich PA, Mancuso L, Kliot D, Wolk DA, Das SR, Mancuso L, Kliot D, Wolk DA, Pluta JB, Das SR, Wisse LEM, Avants BB, Yushkevich PA, Ding SL, Wang H, Manjón JV, **e L, Ding SL (2017) Multi-template analysis of human perirhinal cortex in brain MRI: explicitly accounting for anatomical variability. Neuroimage 144:183–202. https://doi.org/10.1016/j.neuroimage.2016.09.070
Boutet C, Chupin M, Lehéricy S, Marrakchi-Kacem L, Epelbaum S, Poupon C, Wiggins C, Vignaud A, Hasboun D, Defontaines B, Hanon O, Dubois B, Sarazin M, Hertz-Pannier L, Colliot O (2014) Detection of volume loss in hippocampal layers in Alzheimer’s disease using 7 T MRI: a feasibility study. NeuroImage Clin 5:341–348. https://doi.org/10.1016/j.nicl.2014.07.011
Kerchner GA, Deutsch GK, Zeineh M, Dougherty RF, Saranathan M, Rutt BK (2012) Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer’s disease. Neuroimage 63:194–202. https://doi.org/10.1016/j.neuroimage.2012.06.048
Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, Kantarci K, Boeve BF, Pandey MK, Bruinsma T, Knopman DS, Jones DT, Petrucelli L, Cook CN, Graff-Radford NR, Dickson DW, Petersen RC, Jack CR, Murray ME (2016) An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 4:1–19. https://doi.org/10.1186/s40478-016-0315-6
Lladó A, Tort-Merino A, Sánchez-Valle R, Falgàs N, Balasa M, Bosch B, Castellví M, Olives J, Antonell A, Hornberger M (2018) The hippocampal longitudinal axis—relevance for underlying tau and TDP-43 pathology. Neurobiol Aging 70:1–9. https://doi.org/10.1016/j.neurobiolaging.2018.05.035
Wolk DA, Das SR, Mueller SG, Weiner MW, Yushkevich PA (2017) Medial temporal lobe subregional morphometry using high resolution MRI in Alzheimer’s disease. Neurobiol Aging 49:204–213. https://doi.org/10.1016/j.neurobiolaging.2016.09.011
King A, Bodi I, Nolan M, Troakes C, Al-Sarraj S (2015) Assessment of the degree of asymmetry of pathological features in neurodegenerative diseases. What is the significance for brain banks? J Neural Transm 122:1499–1508. https://doi.org/10.1007/s00702-015-1410-8
Stefanits H, Budka H, Kovacs GG (2012) Asymmetry of neurodegenerative disease-related pathologies: a cautionary note. Acta Neuropathol 123:449–452
Montine TJ, Monsell SE, Beach TG, Bigio EH, Bu Y, Cairns NJ, Frosch M, Henriksen J, Kofler J, Kukull WA, Lee EB, Nelson PT, Schantz AM, Schneider JA, Sonnen JA, Trojanowski JQ, Vinters HV, Zhou XH, Hyman BT (2016) Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer’s disease. Alzheimer’s Dement 12:164–169. https://doi.org/10.1016/j.jalz.2015.07.492
Kovacs GG, **e SX, Robinson JL, Lee EB, Smith DH, Schuck T, Lee VMY, Trojanowski JQ (2018) Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol Commun 6:50. https://doi.org/10.1186/s40478-018-0552-y
Dugger BN, Dickson DW (2017) Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol 9:a028035
Perry G, Kawai M, Tabaton M, Onorato M, Mulvihill P, Richey P, Morandi A, Connolly JA, Gambettil P (1991) Neuropil threads of Alzheimer’s disease show a marked alteration of the normal cytoskeleton. J Neurosci 17:1746–1755
Alegro M, Chen Y, Ovando D, Heinsen H, Eser R, Tosun D, Grinberg LT (2019) Deep learning based large-scale histological tau protein map** for neuroimaging biomarker validation in Alzheimer’s disease. bioRxiv. https://doi.org/10.1101/698902
Tward D, Brown T, Kageyama Y, Patel J, Hou Z, Mori S, Albert M, Troncoso J, Miller M (2020) Diffeomorphic registration with intensity transformation and missing data: application to 3D digital pathology of Alzheimer’s disease. Front Neurosci 14:52. https://doi.org/10.3389/fnins.2020.00052
Acknowledgements
We gratefully acknowledge the tissue donors and their families. We also thank all the staff at the National Disease Research Interchange brain bank and at Center for Neurodegenerative Research (University of Pennsylvania) and Human Neuroanatomy Lab (University of Castilla-La Mancha—UCLM) for performing the autopsies and making the tissue available for this project, as well as Sonia Herreros and personnel of the Department of Pathology, Albacete University Hospital.
Funding
This work was supported in part by the National Institute of Health (Grants R01 EB017255, R01 AG056014, P30 AG010124, R01 AG054519, AG017586), a UCLM Travel Grant (to R.I.), a UCLM research grant to Human Neuroanatomy Laboratory and an Alzheimer’s Association Grant (AARF-19-615258) (to L.E.M.W).
Author information
Authors and Affiliations
Contributions
SR, LEMW, DAW, PAY, JAD, MG, JQT, SRD designed research; SR, LEMW, RI, LX, SL, MLB, DJI, EBL, MDT, KP, JLR, TS, EAP, MMIO, MMAJ, MM, FJMR, MPMR, SCS, JCDG, CRP, MCP and PAY performed research; SR, LEMW, DAW, SRD, LX and PAY, RI interpreted data and discussed results; SR and PAY analyzed data and wrote the paper. PAY was the principal designer and coordinator of the study and overviewed collection, analysis, and interpretation of the study data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Human brain specimens were obtained in accordance with the University of Pennsylvania Institutional Review Board guidelines, and the Ethical Committee of UCLM. Where possible, pre-consent during life and, in all cases, next-of-kin consent at death was given.
Consent for publication
All authors have reviewed the contents of the manuscript being submitted, approved of its contents and validated the accuracy of the data and consented to publication.
Competing interests
D.A.W has received grant support from Merck, Biogen, and Eli Lilly/Avid. D.A.W received consultation fees from Neuronix and is on the DSMB for a clinical trial run by Functional Neuromodulation. L.X. received personal consultation fees from Galileo CDS, Inc. J.Q.T. may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is co-Inventor and he received revenue from the sale of Avid to Eli Lily as co-inventor on imaging related patents submitted by the University of Pennsylvania.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary methods and results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ravikumar, S., Wisse, L.E.M., Lim, S. et al. Ex vivo MRI atlas of the human medial temporal lobe: characterizing neurodegeneration due to tau pathology. acta neuropathol commun 9, 173 (2021). https://doi.org/10.1186/s40478-021-01275-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-021-01275-7