Abstract
Heart disease remains the leading cause of mortality globally, so further investigation is required to identify its underlying mechanisms and potential targets for treatment and prevention. Mitsugumin 53 (MG53), also known as TRIM72, is a TRIM family protein that was found to be involved in cell membrane repair and primarily found in striated muscle. Its role in skeletal muscle regeneration and myogenesis has been well documented. However, accumulating evidence suggests that MG53 has a potentially protective role in heart tissue, including in ischemia/reperfusion injury of the heart, cardiomyocyte membrane injury repair, and atrial fibrosis. This review summarizes the regulatory role of MG53 in cardiac tissues, current debates regarding MG53 in diabetes and diabetic cardiomyopathy, as well as highlights potential clinical applications of MG53 in treating cardiac pathologies.
Similar content being viewed by others
Introduction
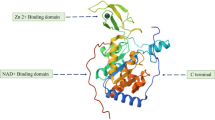
Mitsugumin-53 (MG53), also known as TRIM72, is a cell membrane repair protein that is part of the tripartite motif family of proteins. Similar to other proteins in the TRIM family, MG53 contains the prototypical tripartite motif that includes ring, B-box, and coiled-coil moieties, as well as a SPRY domain at the carboxy terminus [1,2,3]. It is a striated muscle protein, which is highly expressed in skeletal muscles and to a lesser extent cardiac muscles.
Following acute membrane damage, MG53 senses an oxidized intracellular environment and forms an oxidation-dependent oligomerization repair complex by tethering to phosphatidylserine domains present on intracellular vesicles and in the inner aspect of the plasma membrane [4]. A local elevation of Ca2+ enables MG53-tethered intracellular vesicles to fuse with the disrupted plasma membrane, leading to the formation of a repair patch [4]. This process is also facilitated by the interaction of MG53 with muscle specific proteins dysferlin and caveolin-3 (Cav3) [5]. MG53 knock out (KO) mice show progressive myopathy and reduced exercise capacity that is associated with a defect in its membrane repair capability [4].
In addition to its function in skeletal muscle, MG53 has been shown to have protective effects on various forms of cardiac muscle injury. Since cardiomyocytes are terminally differentiated cells with limited self-renewal capacity, and membrane rupture is a major cause of cardiomyocyte cell death following injury, membrane repair is a necessary process for preserving cardiomyocyte viability [6]. In this review, we summarize the biological function of MG53 with its potential mechanisms in cardiac tissue (Fig. 1), discuss current debates regarding the role of MG53 in diabetic cardiomyopathy (Table 1), and potential clinical applications of recombinant MG53 protein in the management and treatment of heart diseases (Table 2).
Beneficial effects of MG53 in heart disease
Cardioprotective effects after ischemia/reperfusion injury
Ischemic preconditioning (IPC) was first reported in 1986 by Murry et al. and is an intrinsic process through which repeated short episodes of ischemia are instituted to protect the myocardium against subsequent ischemic insults [7]. Cardiac ischemia is modelled in vitro through the application of hypoxic and oxidative stress. Ischemia/reperfusion (I/R) in mouse hearts and hypoxia/oxidative stress in neonatal rat cardiomyocytes have been associated with a downregulation of MG53. IPC can prevent IR-induced decrease in MG53 expression [8]. MG53 KO mice lack IPC-mediated cardioprotection as evidenced by a failure of IPC to reduce IR-induced myocardial infarct size. IPC suppressed IR-induced infarction in wild type (WT) mouse hearts whereas overexpression of GFP-MG53 fusion protein reduced hypoxia- or H2O2-induced cell death [8]. However, adenovirus-mediated shRNA targeting MG53 downregulated MG53 expression in rat cardiomyocytes, exacerbating hypoxia-induced cell death and eliminating the protective effect of GFP-MG53 overexpression [8].
IPC activates the reperfusion injury salvage kinase (RISK) and survivor activating factor enhancement (SAFE) pathways to protect the heart against IR injury. The RISK pathway consists of PI3K-Akt-GSK3β and ERK1/2 signaling events (Fig. 1), whereas the SAFE pathway involves activation of tumor necrosis factor-α (TNF-α) and the JAK-STAT3 axis [9,10,11,12]. Overexpression of MG53 significantly increased phosphorylation levels of several key pro-survival kinases including Akt, GSK3β, and ERK1/2 over their respective controls [13,14,15,16]. However, IPC did not enhance the phosphorylation levels of these key kinases in MG53 KO mouse hearts [8]. This suggests that IPC-induced elevation of PI3K-Akt-GSK3 and ERK1/2 signals is MG53 dependent and that suppression of either pathway fully prevents IPC-induced cardioprotection (Fig. 1). Contrastingly, MG53 has not been demonstrated to activate the SAFE pathway [17].
Interestingly, a recent study from ** physiological functions in insulin signaling capabilities [58]. Results from a proteomic study [59] assessed IRS-1 protein interactions in skeletal muscles from normal individuals, obese insulin-resistant nondiabetic control subjects, and patients with type 2 diabetes, before and after insulin infusion. They failed to identify any changes in MG53 protein interaction with IRS-1 across all groups after insulin infusion [59].
Furthermore, a recent published paper from our group [60] revealed lower serum MG53 levels in db/db mice compared with WT littermates (Table 1). Either whole-body knockout of MG53 or sustained increase of MG53 in circulation did not affect insulin signaling and glucose handling in db/db mice. Rats receiving the daily intravenous (IV) treatment of rhMG53 did not have adverse effects on glucose handling [60]. Interestingly, a recent study from ** of proteins released during necrosis and apoptosis from cultured neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2014;306:C639-647." href="/article/10.1186/s13578-021-00629-x#ref-CR67" id="ref-link-section-d6318297e1652">67]. MG53 was found to have a time-dependent relative elevated expression after inducing necrosis by oxidative stress, which supports the potential use of MG53 as a marker for necrotic cellular injury. Using an in vivo murine model, Liu et al. demonstrated a low circulating level of MG53 in the blood at baseline which increased in a dose-dependent fashion after an MI [68]. A recent study showed that the serum level of MG53 was elevated in patients with stable cardiovascular disease and reach to the highest level in patients with an acute MI [69]. These findings suggest MG53 may be a potential biomarker of myocardial injury.
Cardioprotection and therapeutic potential of rhMG53
As discussed above, low levels of endogenous MG53 protein has been found in human hearts, which emphasizes the physiological significance of circulating MG53 for cardioprotection. It has been demonstrated that MG53 can be secreted from skeletal muscle in response to exercise and muscle contraction, and that MG53 circulates in the bloodstream under physiological conditions [70, 71]. Pharmacokinetic (PK) and toxicology assessments support the safety of repetitive IV administration of rhMG53 in Beagle dogs [71]. Studies in mice reported no observable toxic effects with long-term IV or subcutaneous (SQ) administration of rhMG53 [70, 71]. Therefore, therapeutic approaches to modulate MG53 function or systemic administration of rhMG53 protein can potentially act as safe biological interventions to treat cardiac pathologies. As a therapeutic protein, rhMG53 has several practical attributes. First, since native MG53 is present and expressed constitutively in humans and the protein can be found normally in circulation, there are minimal toxicological and immunological concerns for using rhMG53 as a therapeutic protein. Second, rhMG53 can be purified using E. coli fermentation, be stored as a lyophilized powder long term at room temperature, and remain soluble and biologically active upon reconstitution [70].
Administration of recombinant human MG53 (rhMG53) mitigated sepsis-induced myocardial dysfunction in rats evidenced by the improved survival rate with increased cardiac function, and reduced oxidative stress, inflammation, and myocardial apoptosis via the elevation of PPARα signal pathway (Table 2) [44]. Given the role of MG53 in IPC and PostC, therapeutic rhMG53 could potentially be used to enhance the protective effects of these maneuvers (Table 2) [18]. However, because IPC can only protect against IR injury before the occurrence of a severe ischemic episode, this limits the practical application of IPC in the clinical setting of an MI [7]. Because PostC is a series of brief ischemia and reperfusion cycles applied after the onset of reperfusion, PostC may have more practical clinic applications [72].
Liu et al. used several different animal models to identify whether rhMG53 can play a therapeutic role in treating IR injury. They demonstrated that the administration of rhMG53, either before ischemia or after reperfusion, decreased infarct size in these various in vivo models (Table 2) [68]. Furthermore, they provided evidence demonstrating that rhMG53 preferentially targets infarcted tissue and upregulates phospho-AKT and phosphor-GSK3β supporting the mechanism that rhMG53 concentrates at the site of injury by binding phosphatidylserine [68, 70]. Importantly, animals with the daily administration of rhMG53 did not exhibit side effects on glucose handling (Table 2) [60].
The administration of rhMG53 can come in various forms. The protein can potentially be directly injected at or in the site of myocardial injury to promote repair or attenuate injury. rhMG53 could also be administered by IV, SQ [70], and intramuscular (IM) injections [73]. Exogenously applied rhMG53 has been shown to be able to recognize sites of injury on the membrane of cardiomyocytes and reduce infarct size [68].
There may be potential for gene therapy applications with adeno-associated virus (AAV)-mediated delivery of MG53. For example, given the essential role of MG53 in maintaining the integrity of muscle membranes, MG53 may be used to treat different forms of muscular dystrophy. MG53 gene therapy may also have a role in treating various forms of cardiomyopathies considering the ability of MG53 to maintain the integrity of cardiomyocyte membranes [22]. He et al. demonstrated the systemic delivery and muscle-specific overexpression of human MG53 gene by recombinant AAV vectors. They reported enhanced membrane repair and improved muscle and heart function with MG53 overexpression in δ-sarcoglycan-deficient TO-2 hamsters, an animal model of muscular dystrophy and congestive heart failure [22].
Conclusions
In addition to its function in skeletal muscle, MG53 appears to play an important role in cardiac muscle as well. Its critical function as a membrane repair protein has been clearly demonstrated. It also appears to be important for mediating the cardioprotective effects of both ischemic preconditioning and post-conditioning after ischemia/reperfusion injury. MG53 may also play a role in the development of atrial fibrosis, which, in turn can promote atrial fibrillation. However, there are still debates on the potential role of MG53 in mediating and even promoting diabetes, diet-induced metabolic disorders, and diabetic cardiomyopathies. The potential utility of MG53 as a diagnostic biomarker and rhMG53 as a clinically relevant of therapeutic protein is promising and warrants further study.
Availability of data and materials
Not applicable.
References
Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of “single protein RING finger” E3 ubiquitin ligases. BioEssays. 2005;27:1147–57.
Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, et al. The tripartite motif family identifies cell compartments. EMBO J. 2001;20:2140–51.
Ponting C, Schultz J, Bork P. SPRY domains in ryanodine receptors (Ca2+-release channels). Trends Biochem Sci. 1997;22:193–4.
Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11:56–64.
Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284:15894–902.
Steenbergen C, Hill ML, Jennings RB. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ Res. 1985;57:864–75.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36.
Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, et al. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 2010;121:2565–74.
Crisostomo PR, Wairiuko GM, Wang M, Tsai BM, Morrell ED, et al. Preconditioning versus postconditioning: mechanisms and therapeutic potentials. J Am Coll Surg. 2006;202:797–812.
Heusch G. No RISK, no … cardioprotection? A critical perspective. Cardiovasc Res. 2009;84:173–5.
Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40.
Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–8.
Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–7.
Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, et al. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94:884–91.
Howes AL, Arthur JF, Zhang T, Miyamoto S, Adams JW, et al. Akt-mediated cardiomyocyte survival pathways are compromised by G alpha q-induced phosphoinositide 4,5-bisphosphate depletion. J Biol Chem. 2003;278:40343–51.
Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase–dependent pathway is cardioprotective. Circ Res. 2002;90:377–9.
Zhang Y, Lv F, ** L, Peng W, Song R, et al. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res. 2011;91:108–15.
Shan D, Guo S, Wu HK, Lv F, ** L, et al. Cardiac ischemic preconditioning promotes MG53 secretion through H2O2-activated protein kinase C-delta signaling. Circulation. 2020;142:1077–91.
Pagliaro P, Penna C. Cardiac postconditioning. Antioxid Redox Signal. 2011;14:777–9.
Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–54.
Wang X, **e W, Zhang Y, Lin P, Han L, et al. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res. 2010;107:76–83.
He B, Tang RH, Weisleder N, **ao B, Yuan Z, et al. Enhancing muscle membrane repair by gene delivery of MG53 ameliorates muscular dystrophy and heart failure in delta-Sarcoglycan-deficient hamsters. Mol Ther. 2012;20:727–35.
Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42.
Masumiya H, Asaumi Y, Nishi M, Minamisawa S, Adachi-Akahane S, et al. Mitsugumin 53-mediated maintenance of K+ currents in cardiac myocytes. Channels (Austin). 2009;3:6–11.
Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Agren MS. Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007;15:2–16.
Cai C, Lin P, Zhu H, Ko JK, Hwang M, et al. Zinc binding to MG53 protein facilitates repair of injury to cell membranes. J Biol Chem. 2015;290:13830–9.
Kohr MJ, Evangelista AM, Ferlito M, Steenbergen C, Murphy E. S-nitrosylation of TRIM72 at cysteine 144 is critical for protection against oxidation-induced protein degradation and cell death. J Mol Cell Cardiol. 2014;69:67–74.
Zhang C, Chen B, Wang Y, Guo A, Tang Y, et al. MG53 is dispensable for T-tubule maturation but critical for maintaining T-tubule integrity following cardiac stress. J Mol Cell Cardiol. 2017;112:123–30.
Zhang B, Zelhof AC. Amphiphysins: raising the BAR for synaptic vesicle recycling and membrane dynamics. Bin-Amphiphysin-Rvsp Traffic. 2002;3:452–60.
Di Maio A, Karko K, Snopko RM, Mejia-Alvarez R, Franzini-Armstrong C. T-tubule formation in cardiacmyocytes: two possible mechanisms? J Muscle Res Cell Motil. 2007;28:231–41.
McNeil P. Membrane repair redux: redox of MG53. Nat Cell Biol. 2009;11:7–9.
Song R, Peng W, Zhang Y, Lv F, Wu HK, et al. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature. 2013;494:375–9.
Yi JS, Park JS, Ham YM, Nguyen N, Lee NR, et al. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nat Commun. 2013;4:2354.
Wang Q, Yu Y, Zhang P, Chen Y, Li C, et al. The crucial role of activin A/ALK4 pathway in the pathogenesis of Ang-II-induced atrial fibrosis and vulnerability to atrial fibrillation. Basic Res Cardiol. 2017;112:47.
Chang SH, Yeh YH, Lee JL, Hsu YJ, Kuo CT, et al. Transforming growth factor-beta-mediated CD44/STAT3 signaling contributes to the development of atrial fibrosis and fibrillation. Basic Res Cardiol. 2017;112:58.
Huber RJ, O’Day DH. Proteomic profiling of the extracellular matrix (slime sheath) of Dictyostelium discoideum. Proteomics. 2015;15:3315–9.
Chen XQ, Zhang DL, Zhang MJ, Guo M, Zhan YY, et al. TRIF promotes angiotensin II-induced cross-talk between fibroblasts and macrophages in atrial fibrosis. Biochem Biophys Res Commun. 2015;464:100–5.
Guo J, Jia F, Jiang Y, Li Q, Yang Y, et al. Potential role of MG53 in the regulation of transforming-growth-factor-beta1-induced atrial fibrosis and vulnerability to atrial fibrillation. Exp Cell Res. 2018;362:436–43.
Zhao JQ, Lei H. Tripartite motif protein 72 regulates the proliferation and migration of rat cardiac fibroblasts via the transforming growth factor-beta signaling pathway. Cardiology. 2016;134:340–6.
Chen X, Su J, Feng J, Cheng L, Li Q, et al. TRIM72 contributes to cardiac fibrosis via regulating STAT3/Notch-1 signaling. J Cell Physiol. 2019;234:17749–56.
Li H, Duann P, Lin PH, Zhao L, Fan Z, et al. Modulation of wound healing and scar formation by MG53 protein-mediated cell membrane repair. J Biol Chem. 2015;290:24592–603.
Adesanya TMA, Russell M, Park KH, Zhou XY, Sermersheim MA, et al. MG53 protein protects aortic valve interstitial cells from membrane injury and fibrocalcific remodeling. J Am Heart Assoc. 2019. https://doi.org/10.1161/JAHA.118.009960.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
Han X, Chen DL, Liufu N, Ji FT, Zeng QS, et al. MG53 protects against sepsis-induced myocardial dysfunction by upregulating peroxisome proliferator-activated receptor-alpha. Oxidative Med Cell Longev. 2020;2020:7413693.
Cai C, Masumiya H, Weisleder N, Pan Z, Nishi M, et al. MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem. 2009;284:3314–22.
Liu F, Song R, Feng Y, Guo J, Chen Y, et al. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor alpha. Circulation. 2015;131:795–804.
Wu HK, Zhang Y, Cao CM, Hu X, Fang M, et al. Glucose-sensitive myokine/cardiokine MG53 regulates systemic insulin response and metabolic homeostasis. Circulation. 2019;139:901–14.
Qi J, Yang B, Ren C, Fu J, Zhang J. Swimming exercise alleviated insulin resistance by regulating tripartite motif family protein 72 expression and AKT signal pathway in Sprague-Dawley rats fed with high-fat diet. J Diabetes Res. 2016;2016:1564386.
Reddy SS, Shruthi K, Prabhakar YK, Sailaja G, Reddy GB. Implication of altered ubiquitin-proteasome system and ER stress in the muscle atrophy of diabetic rats. Arch Biochem Biophys. 2018;639:16–25.
Ma H, Liu J, Bian Z, Cui Y, Zhou X, et al. Effect of metabolic syndrome on mitsugumin 53 expression and function. PLoS ONE. 2015. https://doi.org/10.1371/journal.pone.0124128.
Ma LL, Kong FJ, Guo JJ, Zhu JB, Shi HT, et al. Hypercholesterolemia abrogates remote ischemic preconditioning-induced cardioprotection: role of reperfusion injury salvage kinase signals. Shock. 2017;47:363–9.
Ma LL, Zhang FJ, Qian LB, Kong FJ, Sun JF, et al. Hypercholesterolemia blocked sevoflurane-induced cardioprotection against ischemia-reperfusion injury by alteration of the MG53/RISK/GSK3beta signaling. Int J Cardiol. 2013;168:3671–8.
Xu Y, Ma LL, Zhou C, Zhang FJ, Kong FJ, et al. Hypercholesterolemic myocardium is vulnerable to ischemia-reperfusion injury and refractory to sevoflurane-induced protection. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0076652.
Yuan H, Niu Y, Liu X, Yang F, Niu W, et al. Proteomic analysis of skeletal muscle in insulin-resistant mice: response to 6-week aerobic exercise. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0053887.
Zabielski P, Lanza IR, Gopala S, Heppelmann CJ, Bergen HR 3rd, et al. Altered skeletal muscle mitochondrial proteome as the basis of disruption of mitochondrial function in diabetic mice. Diabetes. 2016;65:561–73.
Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–6.
Terauchi Y, Iwamoto K, Tamemoto H, Komeda K, Ishii C, et al. Development of non-insulin-dependent diabetes mellitus in the double knockout mice with disruption of insulin receptor substrate-1 and beta cell glucokinase genes. Genetic reconstitution of diabetes as a polygenic disease. J Clin Invest. 1997;99:861–6.
Laustsen PG, Michael MD, Crute BE, Cohen SE, Ueki K, et al. Lipoatrophic diabetes in Irs1(-/-)/Irs3(-/-) double knockout mice. Genes Dev. 2002;16:3213–22.
Caruso M, Ma D, Msallaty Z, Lewis M, Seyoum B, et al. Increased interaction with insulin receptor substrate 1, a novel abnormality in insulin resistance and type 2 diabetes. Diabetes. 2014;63:1933–47.
Wang Q, Bian Z, Jiang Q, Wang X, Zhou X, et al. MG53 does not manifest the development of diabetes in db/db mice. Diabetes. 2020;69:1052–64.
Ham YM, Mahoney SJ. Compensation of the AKT signaling by ERK signaling in transgenic mice hearts overexpressing TRIM72. Exp Cell Res. 2013;319:1451–62.
Liu W, Wang G, Zhang C, Ding W, Cheng W, et al. MG53, a novel regulator of KChIP2 and Ito, f, plays a critical role in electrophysiological remodeling in cardiac hypertrophy. Circulation. 2019;139(18):2142–56.
Bian Z, Wang Q, Zhou X, Tan T, Park KH, et al. Sustained elevation of MG53 in the bloodstream increases tissue regenerative capacity without compromising metabolic function. Nat Commun. 2019;10:4659.
Zhu H, Hsueh W, Whitson BA. Letter by Zhu et al regarding article, “Glucose-sensitive myokine/cardiokine mg53 regulates systemic insulin response and metabolic homeostasis.” Circulation. 2019;140:e186–7.
Philouze CTS, Cremers B, Caliez A, Lamarche G, Bernard C, Provost N, Delerive P. MG53 is not a critical regulator of insulin signaling pathway in skeletal muscle. PLoS ONE. 2021;16(2):e0245179.
Lemckert FA, Bournazos A, Eckert DM, Kenzler M, Hawkes JM, et al. Lack of MG53 in human heart precludes utility as a biomarker of myocardial injury or endogenous cardioprotective factor. Cardiovasc Res. 2016;110:178–87.
Marshall KD, Edwards MA, Krenz M, Davis JW, Baines CP. Proteomic map** of proteins released during necrosis and apoptosis from cultured neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2014;306:C639-647.
Liu J, Zhu H, Zheng Y, Xu Z, Li L, et al. Cardioprotection of recombinant human MG53 protein in a porcine model of ischemia and reperfusion injury. J Mol Cell Cardiol. 2015;80:10–9.
**e HY, Wang YQ, Zhu TQ, Feng S, Yan ZJ, et al. Serum MG53/TRIM72 is associated with the presence and severity of coronary artery disease and acute myocardial infarction. Front Physiol. 2020;11:1658.
Weisleder N, Takizawa N, Lin P, Wang X, Cao C, et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4:139ra185.
Duann P, Li H, Lin P, Tan T, Wang Z, et al. MG53-mediated cell membrane repair protects against acute kidney injury. Sci Transl Med. 2015;7:279ra236.
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579-588.
Corona BT, Garg K, Roe JL, Zhu H, Park KH, et al. Effect of recombinant human MG53 protein on tourniquet-induced ischemia-reperfusion injury in rat muscle. Muscle Nerve. 2014;49:919–21.
Acknowledgements
Not applicable
Funding
PL was supported by a grant from NIGMS (5K08GM126315), CC was supported by an AHA grant (18TPA34170188), and JM was supported by NIH grants (AR061385, AR070752 and AG056919).
Author information
Authors and Affiliations
Contributions
WZ, DB: Drafted the manuscript. CC: made the figure and Tables. PL, CC, JM: supervised, reviewed, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All authors have their consent to participate.
Consent for publication
All authors agree to publish this manuscript.
Competing interests
JM hold equity interest in TRIM-edicine, Inc., a university spin-off biotechnology company that develops MG53 for regenerative medicine application. Rutgers University and Ohio State University own the Intellectual property related to MG53. All the other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhong, W., Benissan-Messan, D.Z., Ma, J. et al. Cardiac effects and clinical applications of MG53. Cell Biosci 11, 115 (2021). https://doi.org/10.1186/s13578-021-00629-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-021-00629-x