Abstract
Background
The efficacy of human umbilical cord mesenchymal stem cell (hUC-MSC) transplantation in treating systemic lupus erythematosus (SLE) has been confirmed by small-scale clinical trials. However, these trials focused on severe or refractory SLE, while few studies focused on mild SLE. Therefore, this study focused on the therapeutic effects of hUC-MSC transplantation in early-stage or mild MRL/lpr lupus model mice.
Methods
Commercially available hUC-MSCs were transplanted into 8-week-old MRL/lpr mice by tail vein injection. Flow cytometry was used to analyze B cells and their subsets in the peripheral blood. Further, plasma inflammatory factors, autoantibodies, and plasma biochemical indices were detected using protein chip technology and ELISA kits. In addition, pathological staining and immunofluorescence were performed to detect kidney injury in mice.
Results
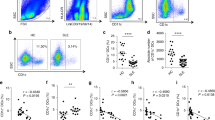
hUC-MSC transplantation did not affect the mice’s body weight, and both middle and high dose hUC-MSC transplantation (MD and HD group) actually reduced spleen weight. hUC-MSC transplantation significantly decreased the proportion of plasmablasts (PB), IgG1− PB, IgG1+ PB, IgG1+ memory B (MB) cells, IgG1+ DN MB, and IgG1+ SP MB cells. The hUC-MSC transplantation had significantly reduced plasma levels of inflammatory factors, such as TNF-α, IFN-γ, IL-6, and IL-13. Pathological staining showed that the infiltration of glomerular inflammatory cells was significantly reduced and that the level of glomerular fibrosis was significantly alleviated in hUC-MSC-transplanted mice. Immunofluorescence assays showed that the deposition of IgG and IgM antibodies in the kidneys of hUC-MSC-transplanted mice was significantly lower than in the control.
Conclusion
hUC-MSC transplantation could inhibit the proliferation and differentiation of peripheral blood B cells in the early-stage of MRL/lpr mice, thereby alleviating the plasma inflammatory environment in mice, leading to kidney injury remission. The study provides a new and feasible strategy for SLE treatment.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the aberrant activation of B cells and autoantibody production [1]. The pathogenic autoantibodies combine with the corresponding autoantigens; they are then deposited in the skin, joints, glomeruli, and other parts of the body leading to multiple organs and system damage (e.g., lupus nephritis; LN) [2,Blood sample collection and processing Blood sample collection was performed by cardiac puncture after anesthesia at 14 weeks old. The blood samples were collected with EDTA anticoagulant tube and centrifuged at 3000 rpm for 10 min to obtain the plasma, the precipitation is blood cells for flow cytometry (FCM) analysis, and the supernatant is plasma which was immediately stored at − 80 °C. After centrifugation of mice's peripheral blood, red blood cells were lysed using a lysis buffer (BD PharmLyse Lysis Buffer; BD Biosciences, San Diego, CA, USA) to obtain a single-cell suspension. The single-cell suspension was incubated with Purified anti-mouse CD16/32 Antibody (Catalog No. 158002, BioLegend, San Diego, CA, USA) to block nonspecific antibody binding. Subsequently, a cocktail of fluorescence-conjugated antibodies, including CD19, CD138, and PD-L2, was added for staining. Following staining, the cells were fixed with 4% PFA and analyzed using the FACS Celesta™ Flow Cytometer (BD Biosciences, San Diego, CA, USA). The analysis of B cell subsets and the gate scheme in flow cytometry were performed according to previous reports (Fig. 3A) [23, 24]. Furthermore, to evaluate cell surface PD-L1 expression, hUC-MSCs were stained with Alexa Fluor 647-conjugated PD-L1 antibody (Additional file 1: Fig. S1A). All flow cytometry data were analyzed using FlowJo 10.8.1 software (FlowJo, Ashland, OR, USA). For mice lymphocyte-derived single cells, the samples were down-sampled to 30,000 cells per sample. The detailed list of fluorescence-conjugated antibodies used for flow cytometry can be found in Additional file 2: Table S1.
Plasma levels of Antinuclear Antibodies (ANA), anti-dsDNA antibodies, and complement 3 (C3) were measured using a Mouse ANA Total Ig ELISA Kit (Catalog No. 5210, Alpha Diagnostic, San Antonio, Texas, USA), a Mouse anti-dsDNA Antibodies Total Ig ELISA Kit (Catalog No. 5110, Alpha Diagnostic, San Antonio, Texas, USA), and a Mouse C3 ELISA Kit (Catalog No. 6270, Alpha Diagnostic, San Antonio, Texas, USA), respectively, according to the manufacturer’s instructions. Plasma levels of inflammatory cytokines, including interferon gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-10, and IL-13, were assayed using a Milliplex® MAP kit (Millipore, Billerica, MA, USA), according to the manufacturer’s recommendations. Plasma levels of TGF-β1 were assayed using a Mouse TGF-β1 ELISA kit (Catalog No. VAL611, Novus Biologicals, USA) according to the manufacturer’s instructions. Plasma biochemical parameters, including albumin/globulin (A/G) ratio, glycocholic acid (CG), triglyceride (TG), and total bile acid (TBA), were measured using an autoanalyzer (Cobas 8000, Roche, Switzerland). The MRL/lpr mice (14 weeks old) fasted for 12 h, and the total body weight was measured before sacrifice. Next, the spleens were dissected and weighed. The spleen index is presented as spleen/body weight. Kidneys were harvested at the time of sacrifice and fixed with 4% paraformaldehyde in PBS. The kidneys were then embedded in paraffin and sectioned (1.5 μm). Sections were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and Masson’s trichrome stain, following a blind assessment by an experienced pathologist. The quantification of renal histopathological changes was performed as previously described in reference [45]. The study co-cultured BM-MSCs with NZB/NZW F1 mouse PC in vitro and found that MSCs enhanced the viability and function of PC and promoted the production of IgG antibodies [45]; however, the mechanism was unclear. These results further illustrate the potential proinflammatory risk of MSCs, and the impact of this change on disease progression remains to be further verified. On the other hand, the evaluation of renal function and renal pathology found that the 24-h urine protein content of lupus mice in the MD and HD groups were lower than the Ctrl. The results of the renal pathological staining and immunofluorescence analysis showed that the renal pathology of mice in the MD and HD groups had an improvement compared to the Ctrl; these results are similar to those of previous reports. Transplantation of BM-MSCs derived from B6 mice into NZB/NZW F1 lupus model mice did not contribute to disease progression and did not affect autoantibody production, proteinuria levels, or mortality, but did improve renal pathology and reduce lymphocytic infiltration [46]. The results demonstrate that MSC transplantation could reduce the deposition of IgG antibodies in the kidney and alleviate kidney damage to a certain extent. However, hUC-MSC transplantation may not have a repair effect on kidney damage. Still, hUC-MSC transplantation in the early-stage of the disease can reduce the risk of kidney injury secondary to SLE. Further, it has a specific preventive and protective effect on the kidney. The reasons for the inefficacy of MSC transplantation are still unclear, and it is a critical question that impedes the clinical application of MSCs. Therefore, it is a promising strategy to maintain MSCs viability and protect against oxidative stress by preconditioning or modification in vitro, such as improvement of culture methods [47], growth factor or cytokine stimulation [48], hypoxia induction [49], and genetic modifications [50, 51] to enhance its immunosuppressive functions [52]. However, these modification strategies are still in the basic experimental stage and require more evidential support and rigorous clinical trials. Therefore, further research is being done to explore the autophagy activation of hUC-MSC transplantation in SLE treatments, as well as trying to modify MSCs from a new perspective to enhance their immunosuppressive effects and reduce proinflammatory side effects.Flow cytometry (FCM)
Enzyme-linked immunosorbent assay (ELISA)
Determination of plasma cytokine levels
Determination of biochemical parameters
Spleen index measurement
Pathology assessment of kidneys
Conclusion
This study comprehensively analyzed the altered frequency of peripheral B cell subsets by FCM. Further, it analyzed inflammatory cytokine plasma levels by protein chip technology and their changes associated with hUC-MSC transplantation in MRL/lpr mice. This study found that hUC-MSC transplantation partially alleviated disease progression and exerted a protective effect on the kidneys, the MD had the best effect, and LD and HD also had a weak effect, but some parameters in the HD group were contrary to expectations, indicating that higher concentration of hUC-MSC transplantation may cause opposite outcomes. Furthermore, the lack of a comparison with advanced lupus mice makes it impossible to determine whether hUC-MSC transplantation is better in the early stages of the disease than in the advanced stages. Therefore, further research is required on the immunotherapy effects of hUC-MSCs modification on SLE and whether hUC-MSC transplantation in the early-stage of the disease can effectively delay disease progression and maintain extended low activity.
Availability of data and materials
The data that supports the fundings of this study are available on request from the authors.
Abbreviations
- A/G ration:
-
Albumin/globulin ratio
- ACR:
-
Albumin/creatinine ratio
- ANA:
-
Anti-nuclear antibodies
- BUN:
-
Blood urea nitrogen
- CG:
-
Glycocholic acid
- Ctrl:
-
Control
- HD:
-
High dose
- hUC-MSCs:
-
Human umbilical cord mesenchymal stem cells
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- LD:
-
Low dose
- MB:
-
Memory B cells
- MD:
-
Middle dose
- P:
-
Inorganic phosphorus
- PB:
-
Plasmablasts
- PC:
-
Plasma cells
- SLE:
-
Systemic lupus erythematosus
- TBA:
-
Total bile acid
- TG:
-
Triglyceride
- TGF-β1:
-
Transforming growth factor-beta l
- TNF-α:
-
Tumor necrosis factor-alpha
- UA:
-
Uric acid
References
Marion TN, Postlethwaite AE. Chance, genetics, and the heterogeneity of disease and pathogenesis in systemic lupus erythematosus. Semin Immunopathol. 2014;36(5):495–517.
Murphy G, Isenberg DA. New therapies for systemic lupus erythematosus - past imperfect, future tense. Nat Rev Rheumatol. 2019;15(7):403–12.
Peng Y, Guo F, Liao S, Liao H, **ao H, Yang L, Liu HF, Pan Q. Altered frequency of peripheral B-cell subsets and their correlation with disease activity in patients with systemic lupus erythematosus: a comprehensive analysis. J Cell Mol Med. 2020;24(20):12044–53.
Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol. 2020;21(6):605–14.
Cheng Y, Li M, Zhao J, Ye Z, Li C, Li X, Zhu P, Wang Z, Zheng Y, Li X, Zhang M, Huang C, Zeng X. Chinese SLE treatment and research group (CSTAR) registry:VIII. Influence of socioeconomic and geographical variables on disease phenotype and activity in Chinese patients with SLE. Int J Rheum Dis. 2018;21(3):716–24.
Pan Q, Li Y, Ye L, Deng Z, Li L, Feng Y, Liu W, Liu H. Geographical distribution, a risk factor for the incidence of lupus nephritis in China. BMC Nephrol. 2014;15:67.
Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, Amoura Z, Yu X, Mok CC, Santiago MB, Saxena A, Green Y, Ji B, Kleoudis C, Burriss SW, Barnett C, Roth DA. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383(12):1117–28.
Ward M, Tektonidou MG. Belimumab as add-on therapy in lupus nephritis. N Engl J Med. 2020;383(12):1184–5.
Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–31.
Ramsköld D, Parodis I, Lakshmikanth T, Sippl N, Khademi M, Chen Y, Zickert A, Mikeš J, Achour A, Amara K, Piehl F, Brodin P, Gunnarsson I, Malmström V. B cell alterations during BAFF inhibition with belimumab in SLE. EBioMedicine. 2019;40:517–27.
Doria A, Zen M, Canova M, Bettio S, Bassi N, Nalotto L, Rampudda M, Ghirardello A, Iaccarino L. SLE diagnosis and treatment: when early is early. Autoimmun Rev. 2010;10(1):55–60.
Doria A, Briani C. Lupus: improving long-term prognosis. Lupus. 2008;17(3):166–70.
Barbado J, Tabera S, Sánchez A, García-Sancho J. Therapeutic potential of allogeneic mesenchymal stromal cells transplantation for lupus nephritis. Lupus. 2018;27(13):2161–5.
Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22(12):2267–77.
Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, Rojo D, Mönckeberg G, Figueroa FE. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19(3):317–22.
Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27(6):1421–32.
Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62(8):2467–75.
Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69(8):1423–9.
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ, Han ZC. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–26.
Miao Z, ** J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30(9):681–7.
Wang D, Niu L, Feng X, Yuan X, Zhao S, Zhang H, Liang J, Zhao C, Wang H, Hua B, Sun L. Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: a 6-year follow-up study. Clin Exp Med. 2017;17(3):333–40.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Wang H, Lu M, Zhai S, Wu K, Peng L, Yang J, **a Y. ALW peptide ameliorates lupus nephritis in MRL/lpr mice. Arthritis Res Ther. 2019;21(1):261.
Zhang X, Meng J, Shi X, Quinet RJ, Davis W, Zakem J, Keshavamurthy C, Patel R, Lobo G, Hellmers L, Ray AN, Rivers LE, Ali H, Posas-Mendoza T, Hille C, You Z. Lupus pathogenesis and autoimmunity are exacerbated by high fat diet-induced obesity in MRL/lpr mice. Lupus Sci Med. 2023;10(1):e000898.
Nielsen DM, Crnic LS. Elevated plus maze behavior, auditory startle response, and shock sensitivity in predisease and in early stage autoimmune disease MRL/lpr mice. Brain Behav Immun. 2002;16(1):46–61.
Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112(9):3543–53.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22.
Li A, Guo F, Pan Q, Chen S, Chen J, Liu HF, Pan Q. Mesenchymal stem cell therapy: hope for patients with systemic lupus erythematosus. Front Immunol. 2021;12:728190.
Munir H, McGettrick HM. Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells Dev. 2015;24(18):2091–100.
El-Jawhari JJ, El-Sherbiny Y, McGonagle D, Jones E. Multipotent mesenchymal stromal cells in rheumatoid arthritis and systemic lupus erythematosus; from a leading role in pathogenesis to potential therapeutic saviors? Front Immunol. 2021;12:643170.
Jang SG, Lee J, Hong SM, Kwok SK, Cho ML, Park SH. Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatology. 2020;59(6):1426–38.
Lee HK, Kim HS, Pyo M, Park EJ, Jang S, Jun HW, Lee TY, Kim KS, Bae SC, Kim Y, Hong JT, Yun J, Han SB. Phorbol ester activates human mesenchymal stem cells to inhibit B cells and ameliorate lupus symptoms in MRL Fas (lpr) mice. Theranostics. 2020;10(22):10186–99.
Wang Y, **ao S, **a Y, Wang H. The therapeutic strategies for SLE by targeting anti-dsDNA antibodies. Clin Rev Allergy Immunol. 2022;63(2):152–65.
Arbitman L, Furie R, Vashistha H. B cell-targeted therapies in systemic lupus erythematosus. J Autoimmun. 2022;132:102873.
Crow MK. Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann Rheum Dis. 2023;82(8):999–1014.
Iwata S, Hajime Sumikawa M, Tanaka Y. B cell activation via immunometabolism in systemic lupus erythematosus. Front Immunol. 2023;14:1155421.
Berti A, Hillion S, Konig MF, Moura MC, Hummel AM, Carmona E, Peikert T, Fervenza FC, Kallenberg CGM, Langford CA, Merkel PA, Monach PA, Seo P, Spiera RF, Brunetta P, St Clair EW, Harris KM, Stone JH, Grandi G, Pers JO, Specks U, Cornec D. Autoreactive plasmablasts after B cell depletion with rituximab and relapses in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2023;75(5):736–47.
Szelinski F, Lino AC, Dörner T. B cells in systemic lupus erythematosus. Curr Opin Rheumatol. 2022;34(2):125–32.
Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rötzschke O, Hui JH, Raghunath M, Stanton LW, Nurcombe V, Cool SM. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33(6):1878–91.
Brennan M, Layrolle P, Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 2020;30(37):1909125.
Tani C, Vagnani S, Carli L, Querci F, Kühl AA, Spieckermann S, Cieluch CP, Pacini S, Fazzi R, Mosca M. Treatment with allogenic mesenchymal stromal cells in a murine model of systemic lupus erythematosus. Int J Stem Cells. 2017;10(2):160–8.
Deng D, Zhang P, Guo Y, Lim TO. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76(8):1436–9.
Alunno A, Montanucci P, Bistoni O, Basta G, Caterbi S, Pescara T, Pennoni I, Bini V, Bartoloni E, Gerli R, Calafiore R. In vitro immunomodulatory effects of microencapsulated umbilical cord Wharton jelly-derived mesenchymal stem cells in primary Sjögren’s syndrome. Rheumatology. 2015;54(1):163–8.
Montanucci P, Alunno A, Basta G, Bistoni O, Pescara T, Caterbi S, Pennoni I, Bini V, Gerli R, Calafiore R. Restoration of t cell substes of patients with type 1 diabetes mellitus by microencapsulated human umbilical cord Wharton jelly-derived mesenchymal stem cells: an in vitro study. Clin Immunol. 2016;163:34–41.
Youd M, Blickarz C, Woodworth L, Touzjian T, Edling A, Tedstone J, Ruzek M, Tubo R, Kaplan J, Lodie T. Allogeneic mesenchymal stem cells do not protect NZBxNZW F1 mice from develo** lupus disease. Clin Exp Immunol. 2010;161(1):176–86.
Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, Casazza S, Uccelli A, Moretta L, Martini A, Traggiai E. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62(9):2776–86.
Yu W, Li S, Guan X, Zhang N, **e X, Zhang K, Bai Y. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2022;133:112646.
Carvalho AÉS, Sousa MRR, Alencar-Silva T, Carvalho JL, Saldanha-Araujo F. Mesenchymal stem cells immunomodulation: the road to IFN-γ licensing and the path ahead. Cytokine Growth Factor Rev. 2019;47:32–42.
Gorgun C, Ceresa D, Lesage R, Villa F, Reverberi D, Balbi C, Santamaria S, Cortese K, Malatesta P, Geris L, Quarto R, Tasso R. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials. 2021;269:120633.
Raziyeva K, Smagulova A, Kim Y, Smagul S, Nurkesh A, Saparov A. Preconditioned and genetically modified stem cells for myocardial infarction treatment. Int J Mol Sci. 2020;21(19):7301.
Xu J, Chen J, Li W, Lian W, Huang J, Lai B, Li L, Huang Z. Additive therapeutic effects of mesenchymal stem cells and IL-37 for systemic lupus erythematosus. J Am Soc Nephrol. 2020;31(1):54–65.
Lee BC, Kang KS. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res Ther. 2020;11(1):397.
Acknowledgments
We acknowledgement Dr. Minjie Zhang, Dr. Hongluan Wu and Dr. Lin Ye for the treatment of animal models.
Funding
This study was supported by National Natural Science Foundation of China (No. 82070757, 82270770) (QP), Guangdong Provincial Key Laboratory of Autophagy and Major Chronic Noncommunicable Diseases (2022B1212030003) (HL), The Stem Cell Preclinical Research Projects of the Affiliated Hospital of Guangdong Medical University (HL), Guangdong Basic and Applied Basic Research Foundation Enterprise Joint Fund (No. 2022A1515220028) (FG), Affiliated Hospital of Guangdong Medical University “Clinical Medicine +” CnTech Co-operation Project (No. CLP2021B007) (FG), Zhanjiang Science and Technology Project (Competitive) (No. 2021A05060) (FG).
Author information
Authors and Affiliations
Contributions
FG, QRP, HL, and QJP conceived and designed the study and wrote the manuscript. FG, QRP, and TC performed most experiments and statistical analyses. SZL, SML, AL, SC, JC, and ZX repeated some experiments and provide experimental support. HS, LY, and CY helped with the revised manuscript preparation and editing manuscript. FG, HL, and QJP supervised the project. All authors reviewed the manuscript and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethics approval for the experiments was obtained from the laboratory animal ethical committee (LAEC) of Guangdong Medical University (license no. GDY2103031). The Ethics approval document entitled “Mechanism study of human umbilical cord mesenchymal stem cells alleviating the progression of lupus nephritis” was approved on August 25, 2021. All experiments were performed following relevant laws and institutional guidelines of the laboratory animal ethical committee (LAEC) of Guangdong Medical University. This study did not involve human subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
A. MSCs have the capability to interact with B cells via the PD-1/PD-L1 pathway. The flow cytometry results demonstrated that MSCs exhibited a PD-L1 positivity rate exceeding 98% (a). In the spleens of 14-week-old MRL/lpr mice, CD19 and PD-1 exhibited colocalization, presenting as yellow fluorescence (b). DAPI emitted blue fluorescence, CD19 emitted red fluorescence, and PD-1 emitted green fluorescence, scale bar: 50 μm. B. Distribution of spleen T cell subsets, including Tfh cells (CXCR5-positive, green fluorescence) (a), Th1 cells (IFN-γ-positive, green fluorescence), Th2 cells (GATA3-positive, green fluorescence), Th17 cells (IL-17-positive, green fluorescence) and Treg cells (FoxP3-positive, green fluorescence). Blue fluorescence represents DAPI staining, scale bar: 50 μm. The number of mice is n=5.
Additional file 2: Table S1.
Antibodies used in the study.
Additional file 3: Figure S2.
hUC-MSC transplantation effects on spleen weight of 14-week-old MRL/lpr mice. Spleen weight ratio of mice in the MSC transplantation groups (MD and HD groups) showed a decrease compared with the Ctrl. (Ctrl: control, LD: low dose, MD: middle dose, HD: high dose). Compare the distance measured by two rulers of different units. The result indicated that the distance measurement was the same. The number of mice is n = 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, F., Pan, Q., Chen, T. et al. hUC-MSC transplantation therapy effects on lupus-prone MRL/lpr mice at early disease stages. Stem Cell Res Ther 14, 211 (2023). https://doi.org/10.1186/s13287-023-03432-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03432-2