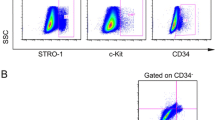
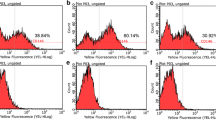
Abstract
Background
Human mesenchymal stem cells from dental pulp (hMSC-DP), including dental pulp stem cells from permanent teeth and exfoliated deciduous teeth, possess unique MSC characteristics such as expression of specific surface molecules and a high proliferation rate. Since hMSC-DP have been applied in numerous clinical studies, it is necessary to establish criteria to evaluate their potency for cell-based therapies.
Methods
We compared stem cell properties of hMSC-DP at passages 5, 10 and 20 under serum (SE) and serum-free (SF) culture conditions. Cell morphology, proliferation capacity, chromosomal stability, surface phenotypic profiles, differentiation and immunoregulation ability were evaluated. In addition, we assessed surface molecule that regulates hMSC-DP proliferation and immunomodulation.
Results
hMSC-DP exhibited a decrease in proliferation rate and differentiation potential, as well as a reduced expression of CD146 when cultured under continuous passage conditions. SF culture conditions failed to alter surface marker expression, chromosome stability or proliferation rate when compared to SE culture. SF-cultured hMSC-DP were able to differentiate into osteogenic, adipogenic and neural cells, and displayed the capacity to regulate immune responses. Notably, the expression level of CD146 showed a positive correlation with proliferation, differentiation, and immunomodulation, suggesting that CD146 can serve as a surface molecule to evaluate the potency of hMSC-DP. Mechanistically, we found that CD146 regulates proliferation and immunomodulation of hMSC-DP through the ERK/p-ERK pathway.
Conclusion
This study indicates that SF-cultured hMSC-DP are appropriate for producing clinical-grade cells. CD146 is a functional surface molecule to assess the potency of hMSC-DP.
Similar content being viewed by others
Introduction
Mesenchymal stem cells (MSCs) have been used in clinics to treat a variety of human diseases [1,2,3,4]. MSCs can be isolated from multiple tissues, such as bone marrow, umbilical cord tissue, adipose tissue and dental pulp [5,6,7]. The minimal criteria for MSC identification were established by the International Society for Cellular Therapy (ISCT) in 2006 [8]. However, the standards for the quality assessment of MSCs from specific tissue resources have not yet been reported.
Human mesenchymal stem cells from dental pulp (hMSC-DP) have been isolated and extensively studied [9, 10]. Their superior proliferation, multi-differentiation, and immunomodulatory capacities have been reported [11,12,54]. In this study, we found that hMSC-DP at early passages such as P5 show optimal immunoregulation effects in vitro in a T cell coculture system and in vivo in a DSS-induced colitis mouse model. However, continued passaging (to P10 and beyond) reduces their therapeutic capacity for colitis mice, which may relate to their diminished ability to induce T cell apoptosis [28].
CD146 was initially identified as a specific marker of malignant melanoma [55]. Previous studies showed that CD146 is expressed on the surface of human bone marrow MSCs, human umbilical cord-derived MSCs, human adipose tissue-derived MSCs, DPSCs and SHED [36, 42, 56,57,58]. MSCs with high expression of CD146 show elevated osteogenic and immunoregulatory abilities compared to those with low CD146 expression [41, 42, 56]. Here, we found that the expression levels of traditional MSC surface markers, including CD73, CD90 and CD105, fail to reflect the potency of hMSC-DP, while the expression level of CD146 correlates with hMSC-DP capacity for proliferation, differentiation, and immunoregulation [26, 59]. Therefore, we propose CD146 as a functional surface molecule to predict the quality of hMSC-DP.
ERK/p-ERK pathway plays a critical role in regulating proliferation, immunoregulation and differentiation of MSCs [27, 60, 61]. Previous studies showed that CD146 expression is associated with the activation of ERK pathway during epithelial-mesenchymal transition [62] and tumor angiogenesis [63]. In this study, we demonstrated that CD146 maintained cell proliferation and immunomodulation through ERK/p-ERK pathway, but not osteogenic differentiation. Usually, a highly proliferative state is incompatible with differentiation in MSCs. The controversial role of ERK signaling has been discussed in the context of osteogenic differentiation of MSCs [45]. Activation of ERK signaling in human bone marrow-derived MSCs promotes osteogenic differentiation [64, 65], while upregulation of ERK/p-ERK pathway contributes to the suppression of osteogenesis of MSCs [27, 44, 46]. ERK/p-ERK may regulate other pathways, such as PI3-kinase/Akt or P38 pathway, to affect osteogenic differentiation of hMSC-DP [66, 67].
The minimal criteria for defining MSCs proposed by ISCT are quite basic and general [8], but it may fail to totally reflect the comprehensive characteristics of MSCs, such as their trophic activity [68] and immunomodulatory capacity [28]. Therefore, it may be insufficient to predict the potency of MSCs for clinical applications. Moreover, different tissue-derived MSCs may exhibit parent tissue specificity and possess different functional potential [69]. With the improvement of our understanding of the functional and tissue-specific characteristics of MSCs, it is necessary to define the criteria of tissue-specific MSCs for translational precision therapies. To meet upcoming requirements for defining optimal MSCs for clinical application, we propose additional criteria to define the potency of hMSC-DP: (1) Adherence to plastic forming CFU-F in serum-free culture conditions; (2) CD146 expressed by over 30% of cells; (3) Neural differentiation potential. These added criteria aim for standardized identification of hMSC-DP for clinical use. In our study, we found that 10–80% of SHED and 5–70% of DPSCs expressed CD146, which was positively correlated with stem cell function. Some critical capacities of hMSC-DP were significantly decreased with the reduced expression of CD146 at passage 10. The positive rate of CD146 expression detected by flow cytometry was about 30–40% at passage 10 in our study. Therefore, we propose that CD146 can serve as a functional surface molecule to evaluate the potency of hMSC-DP. When CD146 positive rate is above 30%, hMSC-DP can provide optimal therapeutic effect in DDS-induced colitis mouse model.
Conclusion
We explored the physiological and functional status of hMSC-DP in the SF culture system and established minimal criteria to identify the potency of hMSC-DP for potential clinical application. CD146 is a functional surface molecule that reflects the potency of hMSC-DP.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional files.
Abbreviations
- hMSC-DP:
-
Human mesenchymal stem cells from dental pulp
- DPSCs:
-
Postnatal human dental pulp stem cells
- SHED:
-
Stem cells from human exfoliated deciduous teeth
- SE:
-
Serum
- SF:
-
Serum-free
- MSCs:
-
Mesenchymal stem cells
- ISCT:
-
International Society for Cellular Therapy
- SOPs:
-
Standard operation procedures
- FBS:
-
Fetal bovine serum
- EdU:
-
5-Ethynyl-20-deoxyuridine
- PD:
-
Population doublings
- CFU:
-
Colony-forming unit
- WGA:
-
Wheat germ agglutinin
- β-gal:
-
β-Galactosidase
- SA-β-gal:
-
Senescence-associated β-galactosidase
- Runx2:
-
Runt-related transcription factor 2
- ALP:
-
Alkaline phosphatase
- PPARγ:
-
Peroxisome proliferator-activated receptor-γ2
- LPL:
-
Lipoprotein lipase
- DSS:
-
Dextran Sulfate Sodium
- DAI:
-
Disease activity index
- HAI:
-
Histological activity index
References
Pittenger MF, Discher DE, Péault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22.
Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–62.
Meng F, Xu R, Wang S, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5(1):172.
Xu X, Jiang W, Chen L, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11(2):e297.
Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, et al. Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology. 2015;71(2):181–97.
Kozlowska U, Krawczenko A, Futoma K, et al. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J Stem Cells. 2019;11(6):347–74.
Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells: current trends and future prospective. Biosci Rep. 2015;35(2):e00191.
Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7.
Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97(25):13625–30.
Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100(10):5807–12.
Botelho J, Cavacas MA, Machado V, et al. Dental stem cells: recent progresses in tissue engineering and regenerative medicine. Ann Med. 2017;49(8):644–51.
Shi X, Mao J, Liu Y. Pulp stem cells derived from human permanent and deciduous teeth: biological characteristics and therapeutic applications. Stem Cells Transl Med. 2020;9(4):445–64.
Sui B, Wu D, **ang L, et al. Dental pulp stem cells: from discovery to clinical application. J Endod. 2020;46(9):S46–55.
Bakopoulou A, About I. Stem cells of dental origin: current research trends and key milestones towards clinical application. Stem Cells Int. 2016;2016:4209891.
Yamada Y, Nakamura-Yamada S, Kusano K, et al. Clinical potential and current progress of dental pulp stem cells for various systemic diseases in regenerative medicine: a concise review. Int J Mol Sci. 2019;20(5):1132.
Xuan K, Li B, Guo H, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10(455):eaaf3227.
Prasad MGS, Ramakrishna J, Babu DN. Allogeneic stem cells derived from human exfoliated deciduous teeth (SHED) for the management of periapical lesions in permanent teeth: two case reports of a novel biologic alternative treatment. J Dent Res Dent Clin Dent Prospects. 2017;11(2):117–22.
Manimaran K, Sankaranarayanan S, Ravi VR, et al. Treatment of osteoradionecrosis of mandible with bone marrow concentrate and with dental pulp stem cells. Ann Maxillofac Surg. 2014;4(2):189–92.
Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16(5):203–9.
** X, Xu Q, Champion K, et al. Endotoxin contamination of apolipoprotein A-I: effect on macrophage proliferation—a cautionary tale. Atherosclerosis. 2015;240(1):121–4.
Tekkatte C, Gunasingh GP, Cherian KM, et al. “Humanized” stem cell culture techniques: the animal serum controversy. Stem Cells Int. 2011;2011:504723.
Spees JL, Gregory CA, Singh H, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9(5):747–56.
Coates DE, Alansary M, Friedlander L, et al. Dental pulp stem cells in serum-free medium for regenerative medicine. J R Soc N Z. 2019;50(1):80–90.
Qu C, Brohlin M, Kingham PJ, et al. Evaluation of growth, stemness, and angiogenic properties of dental pulp stem cells cultured in cGMP xeno-/serum-free medium. Cell Tissue Res. 2020;380(1):93–105.
Iwanaka T, Yamaza T, Sonoda S, et al. A model study for the manufacture and validation of clinical-grade deciduous dental pulp stem cells for chronic liver fibrosis treatment. Stem Cell Res Ther. 2020;11(1):134.
Matsui M, Kobayashi T, Tsutsui TW. CD146 positive human dental pulp stem cells promote regeneration of dentin/pulp-like structures. Hum Cell. 2018;31(2):127–38.
Liu Y, **g H, Kou X, et al. PD-1 is required to maintain stem cell properties in human dental pulp stem cells. Cell Death Differ. 2018;25(7):1350–60.
Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544–55.
Haasters F, Prall WC, Anz D, et al. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat. 2009;214(5):759–67.
Bray M-A, Singh S, Han H, et al. Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat Protoc. 2016;11(9):1757–74.
Zhao H, Darzynkiewicz Z. Biomarkers of cell senescence assessed by imaging cytometry. Methods Mol Biol. 2013;965:83–92.
Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98(14):7841–5.
Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67(19):9142–9.
Lange C, Cakiroglu F, Spiess AN, et al. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213(1):18–26.
Wang Z, Xu Q, Zhang N, et al. CD146, from a melanoma cell adhesion molecule to a signaling receptor. Signal Transduct Target Ther. 2020;5(1):148.
Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704.
Mayo V, Sawatari Y, Huang CY, et al. Neural crest-derived dental stem cells—where we are and where we are going. J Dent. 2014;42(9):1043–51.
Li D, Zou X-Y, El-Ayachi I, et al. Human dental pulp stem cells and gingival mesenchymal stem cells display action potential capacity in vitro after neuronogenic differentiation. Stem Cell Rev Rep. 2019;15(1):67–81.
Anoop M, Datta I. Stem cells derived from human exfoliated deciduous teeth (shed) in neuronal disorders: a review. Curr Stem Cell Res Ther. 2021;16(5):535–50.
Shuai Y, Liao L, Su X, et al. Melatonin treatment improves mesenchymal stem cells therapy by preserving stemness during long-term in vitro expansion. Theranostics. 2016;6(11):1899–917.
Wangler S, Menzel U, Li Z, et al. CD146/MCAM distinguishes stem cell subpopulations with distinct migration and regenerative potential in degenerative intervertebral discs. Osteoarthr Cartil. 2019;27(7):1094–105.
Wu CC, Liu FL, Sytwu HK, et al. CD146+ mesenchymal stem cells display greater therapeutic potential than CD146-cells for treating collagen-induced arthritis in mice. Stem Cell Res Ther. 2016;7:23.
Samsonraj RM, Rai B, Sathiyanathan P, et al. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33(6):1878–91.
Higuchi C, Myoui A, Hashimoto N, et al. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J Bone Miner Res. 2002;17(10):1785–94.
Schindeler A, Little DG. Ras-MAPK signaling in osteogenic differentiation: friend or foe? J Bone Miner Res. 2006;21(9):1331–8.
Li B, Qu C, Chen C, et al. Basic fibroblast growth factor inhibits osteogenic differentiation of stem cells from human exfoliated deciduous teeth through ERK signaling. Oral Dis. 2012;18(3):285–92.
Astori G, Amati E, Bambi F, et al. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther. 2016;7(1):93.
Capelli C, Pedrini O, Valgardsdottir R, et al. Clinical grade expansion of MSCs. Immunol Lett. 2015;168(2):222–7.
Paliwal S, Chaudhuri R, Agrawal A, et al. Human tissue-specific MSCs demonstrate differential mitochondria transfer abilities that may determine their regenerative abilities. Stem Cell Res Ther. 2018;9(1):298.
Ma L, Makino Y, Yamaza H, et al. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS ONE. 2012;7(12):e51777.
Andrukhov O, Behm C, Blufstein A, et al. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: implication in disease and tissue regeneration. World J Stem Cells. 2019;11(9):604–17.
Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74(13):2345–60.
Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507.
Krampera M, Galipeau J, Shi Y, et al. Immunological characterization of multipotent mesenchymal stromal cells—the international society for cellular therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054–61.
Wang Z, Xu Q, Zhang N, et al. CD146, from a melanoma cell adhesion molecule to a signaling receptor. Signal Transduct Target Ther. 2020;5(1):1–5.
Bowles AC, Kouroupis D, Willman MA, et al. Signature quality attributes of CD146(+) mesenchymal stem/stromal cells correlate with high therapeutic and secretory potency. Stem Cells. 2020;38(8):1034–49.
Li X, Guo W, Zha K, et al. Enrichment of CD146(+) adipose-derived stem cells in combination with articular cartilage extracellular matrix scaffold promotes cartilage regeneration. Theranostics. 2019;9(17):5105–21.
Yamaza T, Kentaro A, Chen C, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1(1):5.
Wang XT, Rao NQ, Fang TJ, et al. Comparison of the properties of CD146 positive and CD146 negative subpopulations of stem cells from human exfoliated deciduous teeth. Bei**g Da Xue Xue Bao Yi Xue Ban. 2018;50(2):284–92.
Almalki SG, Agrawal DK. ERK signaling is required for VEGF-A/VEGFR2-induced differentiation of porcine adipose-derived mesenchymal stem cells into endothelial cells. Stem Cell Res Ther. 2017;8(1):113.
Zhang S, Chuah SJ, Lai RC, et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27.
Ma Y, Zhang H, **ong C, et al. CD146 mediates an E-cadherin-to-N-cadherin switch during TGF-β signaling-induced epithelial-mesenchymal transition. Cancer Lett. 2018;430:201–14.
Jiang T, Zhuang J, Duan H, et al. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood. 2012;120(11):2330–9.
Jaiswal RK, Jaiswal N, Bruder SP, et al. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275(13):9645–52.
Murakami J, Ishii M, Suehiro F, et al. Vascular endothelial growth factor-C induces osteogenic differentiation of human mesenchymal stem cells through the ERK and RUNX2 pathway. Biochem Biophys Res Commun. 2017;484(3):710–8.
Baker N, Sohn J, Tuan RS. Promotion of human mesenchymal stem cell osteogenesis by PI3-kinase/Akt signaling, and the influence of caveolin-1/cholesterol homeostasis. Stem Cell Res Ther. 2015;6(1):238.
Xu C, Liu H, He Y, et al. Endothelial progenitor cells promote osteogenic differentiation in co-cultured with mesenchymal stem cells via the MAPK-dependent pathway. Stem Cell Res Ther. 2020;11(1):537.
Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–5.
Rohban R, Pieber TR. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cells Int. 2017;2017:5173732.
Acknowledgements
We thank Mr. Lulu (South China Center of Craniofacial Stem Cell Research, Sun Yat-Sen University) for his kind help in this study.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81700928 to L.M.), the Youth Teacher Training Project of Sun Yat-sen University (17ykpy71 to L.M.), the Pearl River Talent Recruitment Program (2019ZT08Y485 and 2019QN01Y138 to S.S., 2019JC01Y182 to X.K.), the Guangdong Financial Fund for High-Caliber Hospital Construction (174-2018-XMZC-0001-03-0125, D-07 to S.S., D-11 to X.K.), the National Science and Technology Major Project of the Ministry of Science and Technology of China (2018ZX10302207), the Sun Yat-sen University Young Teacher Key Cultivation Project (18ykzd05 to X.K.) and the Natural Science Foundation of Guangdong (2016A030313262 to X.M.).
Author information
Authors and Affiliations
Contributions
LM and ZH contributed to designing the study plan, performing experimental procedures and drafting the manuscript. DW contributed to data acquisition, analysis and interpretation. XK and XM contributed to data analysis and interpretation. SS contributed to the project conception, experimental design, writing manuscript and supervision. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Human samples collection was approved by the Medical Ethics Committee of Hospital of Stomatology, Sun Yat-sen University (Protocol Number: KQEC-2020-055-01). All animal experiments were performed under an institutionally approved protocol for the use of animal research of Sun Yat-sen University (Protocol Number: SYSU-IACUC-2020-000394).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1
Characterization of the morphology of hMSC-DP under SF culture conditions. a-b Cell morphology analysis by high-content imaging. The nuclear area (a) and cell area (b) of DPSCs and SHED are shown in SE and SF culture conditions. n = 3 for each group. c Cellular senescence assay. The percentage of SA-β-gal positive cells was calculated and compared between SE and SF culture conditions. n = 3 ~ 5 for each group. SE, serum; SF, serum-free. Data shown as mean ± SEM. ns, not significant. Fig. S2 Proliferation capacity of hMSC-DP under SF culture conditions. a CFU-F assay. The numbers of colonies of hMSC-DP under SE or SF culture conditions were calculated and compared. n = 3 for each group. b Population doubling scores were calculated and compared between SE and SF culture conditions in DPSCs and SHED groups, independently. n = 3 for each group. c EdU assay. The percentage of EdU-positive cells were calculated and compared between SE and SF culture conditions in DPSCs and SHED groups, independently. n = 3 for each group. Scale bar = 200 μm. SE, serum; SF, serum-free. Data shown as mean ± SEM. *p < 0.05. ns, not significant. Fig. S3 Surface phenotypic profiles and in vitro immunoregulation ability of hMSC-DP. a-b Flow cytometry showed the percentage of CD146-positive hMSC-DP under SE or SF culture conditions at P5, P10 and P20 (a). The percentage of CD146-positive cells was compared between SE and SF culture conditions (b). n = 3 for each group. c The percentage of apoptotic T cells was calculated in SE and SF culture conditions. n = 3 for each group. SE, serum; SF, serum-free. Data shown as mean ± SEM. *p < 0.05. ns, not significant. Fig. S4 Multilineage differentiation of hMSC-DP. a Alizarin red staining assay. The osteogenic capacity was compared between SE and SF culture conditions at P5, P10 and P20. n = 3 for each group. b Oil red O staining assay. The adipogenic capacity of hMSC-DP was compared between SE and SF culture conditions at P5, P10 and P20. n = 3 for each group. Scale bar = 50 μm. SE, serum; SF, serum-free. Data shown as mean ± SEM. **p < 0.01. ns, not significant. Fig. S5 Therapeutic effects of hMSC-DP on experimental colitis. a Disease activity index (DAI) of DPSC- and SHED-treated groups was compared between SE and SF culture conditions at P5, P10 and P20. n = 3 for each group. b The colon length after DPSCs and SHED treatment was compared between SE and SF culture conditions at P5, P10 and P20. n = 3 for each group. c Histological structure was examined by H&E staining. The histological score of each group was compared between SE and SF culture conditions. n = 3 for each group. Scale bar = 100 μm. SE, serum; SF, serum-free. Data shown as mean ± SEM. ns, not significant. Fig. S6 The expression level of CD146 is associated with hMSC-DP properties. a The correlation of CD146 with experimental parameters. b Linear regression plots showed the correlation between CD146 expression and experimental parameters including CFU-F clones, percentage of EdU positive cells, percentage of Alizarin red positive area, percentage of SA-β-gal positive cells, DAI scores, HAI scores and colon length.
Additional file 2
. Table S1. Summary of clinical trials of hMSC-DP.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, L., Huang, Z., Wu, D. et al. CD146 controls the quality of clinical grade mesenchymal stem cells from human dental pulp. Stem Cell Res Ther 12, 488 (2021). https://doi.org/10.1186/s13287-021-02559-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-021-02559-4