Abstract
The inferior vena cava (IVC) is the largest vein in the body, draining blood from the abdomen, pelvis and lower extremities. This pictorial review summarises normal anatomy and embryological development of the IVC. In addition, we highlight a wide range of anatomical variants, acquired pathologies and a common pitfall in imaging of the IVC. This information is essential for clinical decision making and to reduce misdiagnosis.
Similar content being viewed by others
Teaching points
-
IVC anomalies are the result of abnormal persistence or regression of embryological veins.
-
Recurrent pulmonary embolism following routine infrarenal IVC filter placement should raise suspicion of a duplicated IVC.
-
Absent/Interrupted IVC should be suspected in young patients with iliofemoral DVT.
-
IVC variants and dilated collateral veins can be mistaken for malignancy.
-
Tumour thrombus is differentiated from bland thrombus by filling defect enhancement, vessel lumen expansion and contiguity with the mass.
-
Tumour thrombus extent is a key determinant of prognosis and surgical management, particularly in renal cell carcinoma.
Introduction
The inferior vena cava (IVC) is the main conduit for venous return from the pelvis, abdominal viscera and lower extremities. A comprehensive understanding of IVC anatomy, congenital variants and pathology is instrumental to accurate diagnosis and management. In this review, we discuss normal anatomy, embryogenesis and present illustrated cases of congenital anomalies of the IVC including duplicated IVC, left-sided IVC, absent infrarenal IVC and interrupted IVC with azygos continuation. Diseases involving the IVC can be diagnostically challenging, with computed tomography (CT) and magnetic resonance imaging (MRI) playing key roles in characterising malignancy, tumour extent, caval wall invasion and bland thrombus. This information is crucial for staging and surgical planning. Lastly, we discuss the imaging pitfall of mixed artefact masquerading as thrombus.
Normal IVC anatomy
The IVC is a large retroperitoneal vein draining the lower extremities, pelvic and abdominal viscera to the right atrium of the heart. It is typically formed by the confluence of the right and left common iliac veins behind the right common iliac artery at the level of the fifth lumbar vertebra. The IVC ascends along the right side of the vertebral column behind the duodenum, portal vein and liver to pierce the diaphragmatic central tendon at the caval opening at the level of the eighth thoracic vertebra. This is followed by a short intra-thoracic course prior to terminating at the right atrium.
As it ascends, it receives many tributaries including paired third and fourth lumbar veins, the right gonadal vein, paired renal veins, the right suprarenal vein, paired inferior phrenic veins and three hepatic veins. There can be variations to these tributaries such as hepatic accessory veins, the right gonadal vein draining into a right renal vein, or a right lumbar azygos vein draining first and second lumbar veins [1,2,3,4]. Lastly, the azygos venous system supplies collateral circulation between the superior vena cava (SVC) and IVC.
IVC embryology
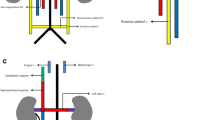
The IVC is formed by a complex process of fusion and subsequent regression of embryological veins, namely the posterior cardinal, subcardinal, supracardinal and vitelline veins. In fourth week of embryological development, the right and left horns of the sinus venosus receive paired common cardinal, umbilical and vitelline veins draining blood from general tissue, the placenta and yolk sac, respectively (Fig. 1a) [5, 6].
Embryological development of the IVC. a The sinus venosus receives paired common cardinal, umbilical and vitelline veins draining blood from general tissue, the placenta and the yolk sac, respectively. b The right hepatocardiac channel (purple) is formed by the right vitelline vein and persists to become the hepatic IVC. Paired subcardinal (red) and supracardinal veins (green) emerge and form multiple anastomotic channels. These include the intersubcardinal anastomoses, suprasubcardinal anastomoses and fusion between the right subcardinal vein (red) and develo** hepatic IVC (purple). As the embryo matures, some of these anastomoses regress. c This illustration highlights vessels that persist to form the mature IVC and its tributaries
During the sixth week of gestation, paired posterior cardinal veins are the dominant vessels carrying blood from the caudal portion of the embryo to the common cardinal vein, and later persist as the mature common iliac veins, anastomosing to form the confluence of left and right common iliac veins in the mature venous system [5, 7]. Proximally, paired vitelline and umbilical veins form the hepatic sinusoidal network, including left and right hepatic veins. As the yolk sac and proximal umbilical veins regress, the cranial aspect of the vitelline veins becomes paired hepatocardiac channels, with the right hepatocardiac channel persisting to become the hepatic segment of the IVC [6].
In the following two weeks of foetal development, paired subcardinal and supracardinal veins emerge as dominant tributaries, forming multiple channels draining into the posterior cardinal veins (Fig. 1b) [5]. The subcardinal veins form ventromedial to the posterior cardinal veins, while the supracardinal veins originate dorsomedial to the posterior cardinal veins [7]. Significant anastomotic networks are formed between paired subcardinal veins (intersubcardinal anastomosis) and between supracardinal and subcardinal vessels (suprasubcardinal anastomoses). The proximal posterior cardinal veins subsequently regress, while metanephric kidneys ascend to connect with the suprasubcardinal anastomoses [8]. The intersubcardinal anastomosis forms the left renal vein and in combination with the suprasubcardinal anastomoses contributes to the renal segment of the IVC [9].
At the same time, the cranial end of the right subcardinal vein forms the suprarenal segment of the IVC and fuses with the develo** hepatic IVC [9]. Gonadal and suprarenal veins are also derived from the subcardinal veins [6]. The caudal left supracardinal vein and left subcardinal veins regress, establishing right-sided dominance. Paired supracardinal veins and their anastomosis extend above the diaphragm to become the azygos and hemiazygos veins [5, 7]. Caudally, the right supracardinal vein persists as the infrarenal IVC, communicating with the paired iliac veins (Fig. 1c) [6].
In summary, the mature IVC is composed of infrarenal (right supracardinal vein), renal (right suprasubcardinal and intersubcardinal anastomoses), suprarenal/infrahepatic (right subcardinal vein) and hepatic (vitelline veins) segments. Posterior cardinal veins persist as paired common iliac veins, whilst the supracardinal veins contribute to the azygos venous system (Fig. 2).
The mature IVC is composed of infrarenal (right supracardinal vein), renal (right suprasubcardinal and intersubcardinal anastomoses), suprarenal (right subcardinal vein) and hepatic (vitelline veins) segments. Renal and suprarenal segments are separated by the dashed line. Posterior cardinal veins persist as paired common iliac veins which join to form the iliac confluence, whilst supracardinal veins contribute to the azygos venous system draining into the superior vena cava. The gonadal and suprarenal veins are derived from subcardinal veins
Anatomical variants
IVC anatomical variants primarily result from abnormal regression or persistence of embryological veins [7]. Although most anomalies are asymptomatic incidental findings, they can cause lower extremity venous insufficiency, deep vein thrombosis, pelvic congestion syndrome and affect planning of vascular procedures [10,11,12,13,14,15,16,17]. The most common anomalies include duplicated IVC, left-sided IVC and interruption of the IVC.
Duplicated IVC
A duplicated IVC is formed by an abnormal persisting left supracardinal vein, resulting in duplicated infrarenal IVC segments (Fig. 3). This variant has a prevalence of 0.2–3% [18]. The common iliac veins typically drain to their respective sides, with the left infrarenal IVC joining the left renal vein, which in turn crosses to join the orthotopic suprarenal IVC. Some studies subclassify this anomaly by differences in calibre of the duplicated infrarenal IVC segments [19]. In addition, a bridging vein may connect the common iliac veins at the inferior origin of the duplicated IVC (Fig. 4) [20]. Rarely, the duplicated segment can continue as the hemiazygos vein and drain directly into the superior vena cava [21, 22]. Case reports of association with retrocaval ureters, horseshoe kidneys and malrotation of the gut have been described [23,24,25]. In this review, we present a rarely described case of duplicated IVC and crossed renal ectopia with both renal veins draining into the orthotopic IVC (Fig. 5) [26]. A duplicated IVC can also be mistaken for adenopathy, or a lymph nodes if its tubular nature is not recognised [16, 27]. Lastly, if this anomaly is not recognised, recurrent pulmonary embolism can occur despite routine infrarenal IVC filter placement [28, 29]. Solutions for this scenario include bilateral infrarenal IVC filter placement, suprarenal IVC filter placement, and steel coil embolisation [30,31,32].
Duplicated IVC with symmetrical caliber. a Contrast-enhanced coronal CT shows a duplicated IVC (asterisk) with symmetrical caliber draining into the suprarenal IVC. Bilateral external iliac veins (arrows) both drain into cavae on their respective sides. b Axial CT image shows the paired IVC trunks (arrows) positioned on either side of the abdominal aorta (arrowhead)
Duplicated IVC with asymmetrical caliber and bridging vein. a Contrasted-enhanced coronal CT shows a duplicated IVC (asterisk) of smaller caliber compared to the orthotopic IVC, that approaches and subsequently drains into the left renal vein (arrow). b Axial CT image shows a bridging vein (arrow) connecting paired common iliac veins (arrowheads) positioned anterior to the L4 vertebral body and posterior to common iliac arteries
Duplicated IVC with right-sided crossed fused renal ectopia. a Coronal CT image shows a duplicated IVC (asterisk) crossing the midline to drain into the right-sided suprarenal IVC. The right renal vein (arrow) and vein of the left ectopic kidney (arrowhead) join before draining into the orthotopic IVC. b Coronal CT demonstrates the right-sided crossed fused renal ectopia and its renal vein (arrow)
Left-sided IVC
Abnormal regression of the right supracardinal vein and persistence of the left supracardinal vein results in a left-sided IVC, with a prevalence of 0.2–0.5% [18]. Bilateral common iliac veins drain into the left-sided IVC which typically course superiorly to join the left renal vein (Fig. 6). Known variants to this configuration include hemiazygos continuation of the left-sided IVC, and an associated retroaortic right renal vein [12, 22, 33]. Identifying a left-sided IVC is important as it can complicate procedures such an abdominal aortic aneurysm repair, left-sided nephrectomy, oblique lumbar fusion or IVC filter placement [7, 34,35,36,37].
Left-sided IVC. a Coronal CT image shows a non-enhanced left-sided IVC (asterisk) draining into an enhanced left renal vein which connects to the hepatic IVC. b Coronal CT image of a second case demonstrates a typical confluence of common iliac veins (yellow asterisk) forming the infrarenal IVC on the left side of the aorta (white asterisk) to join the left renal vein (arrow) which drains into the orthotopic IVC. The proximal right renal vein (arrowhead) is also pictured draining into the suprarenal IVC
Absent infrarenal IVC
An absent infrarenal IVC, also known as interruption of the infrarenal IVC or infrarenal agenesis of IVC with azygos continuation, is postulated to be caused by acquired intrauterine or perinatal venous thrombosis, rather than failure of embryonic vein development [7, 38, 39]. This leads to failure of posterior cardinal and supracardinal vein development, resulting in external and internal iliac veins draining into the azygos–hemiazygos system via ascending lumbar veins, and a preserved suprarenal IVC segment (Fig. 7) [39, 40]. It is a rarely described anomaly with unknown incidence [11, 39, 41, 42]. Affected patients are at risk of develo** lower extremity venous insufficiency, deep vein thrombosis, varicose veins and pelvic congestion syndrome [10,11,12,13,14,15]. In the absence of adequate flow through ascending lumbar veins and the azygos system, other collateral pathways can form involving abdominal wall, pelvic, gonadal and retroperitoneal vessels (Fig. 8) [13].
Absent infrarenal IVC with azygos continuation. Paired common iliac veins continue cranially as bilateral ascending lumbar veins. a Contrast-enhanced coronal CT demonstrates the caudal end of the IVC (asterisk). b Coronal CT shows the right ascending lumbar vein (arrow) approaching and subsequently draining into the IVC (asterisk). Tortuous paravertebral collateral veins (arrowhead) are present adjacent to the aorta. c Axial CT image with left renal vein (arrow) crossing anterior to the aorta to drain into the renal IVC segment (asterisk). Right renal vein (arrowhead) also approaches renal IVC segment. d Axial CT shows prominent bilateral ascending lumbar veins (arrowheads) and a distended paravertebral collateral (arrow) positioned left of the aorta
Absent infrarenal IVC with multiple collateral pathways. Contrast-enhanced delayed venous phase on a CT abdomen and pelvis demonstrates prominent superficial abdominal, epigastric and gonadal veins draining the lower extremities. a Coronal CT image shows paired renal veins (arrowheads) draining into the caudal end of the IVC (asterisk). An aortic stent (arrow) is visualised inferior to the left renal vein. b Axial CT shows prominent external pudendal veins (arrowheads), and the left femoral vein (asterisk) receiving a distended superficial inferior epigastric vein (arrow). c Axial CT image with a distended left gonadal vein (asterisk). Small calibre unenhanced infrarenal IVC (arrow) is suggestive of chronic occlusion. Enhanced right inferior epigastric and abdominal wall veins are also visualised (arrowhead). d Axial CT demonstrates bilateral enlarged pelvic veins (arrows)
Interrupted IVC with azygos continuation
Interrupted IVC with azygos continuation classically refers to interruption of the suprarenal/infrahepatic segment and occurs due to failure of the right subcardinal vein to anastomose with the vitelline vein [7, 43]. An interrupted IVC has been described in various ways with ‘interrupted’ being interchanged for absence, anomalous or agenesis [13, 22, 44]. The suprarenal IVC reroutes to drain via the azygos vein, while the hepatic IVC only receives the hepatic veins. It carries a prevalence of 0.6% and is classically associated with polysplenia, cardiovascular malformations and situs anomalies [7, 16, 45]. Like an absent infrarenal IVC, an interrupted IVC without adequate collateral pathways can similarly result in vascular problems such as deep vein thrombosis and venous insufficiency. An enlarged azygos vein can be misinterpreted as retrocrural lymphadenopathy or a right paratracheal mass, while a distended hemiazygos vein may simulate a left-sided mediastinal mass [43, 46, 47]. Prominent collateral vessels can also be mistaken for paraspinal masses (Fig. 9) [42]. Preoperative awareness of this anomaly is important prior to thoracic and cardiopulmonary bypass surgery [48, 49].
Distended collateral vein mistaken for mass in context of incidentally detected suprarenal IVC stenosis. The 54-year-old man had no previous history of IVC filter insertion or intrabdominal surgery. a Contrast-enhanced sagittal CT shows a pseudomass (arrow) immediately posteroinferior to complete stenosis (arrowhead) of the suprarenal IVC, with caudal segments of the IVC (asterisk) preserved. A small focus of calcification is present at the level of the stenosis. b Axial CT shows preferential blood flow through markedly distended azygos and hemiazygos veins (arrows). c Axial MR T1 VIBE image shows a well-defined lobulated mass (arrow) with venous enhancement. d Axial MR T2 image of the mass (asterisk) shows high T2 signal in kee** with slow venous flow. It communicates posteriorly with the right ascending lumbar vein (yellow arrowhead) and medially with a right paravertebral collateral vein (white arrowhead)
Koc et al. uses a straightforward nomenclature that identifies all anomalies with absent or interrupted segments as ‘interrupted IVC’, followed by the level of interruption and associated collaterals, i.e. ‘interrupted IVC (suprarenal level) with azygos continuation’ [12]. In this review, we report a rarely described case of interrupted IVC of the renal segment with azygos continuation (Fig. 10) [13]. Instead of draining directly into the IVC, the left renal vein drains into a tortuous paravertebral collateral that eventually joins the hemiazygos vein, while the right renal vein continues to drain directly into the IVC. Paired common iliac veins continue as bilateral ascending lumbar veins to join a distended azygos–hemiazygos system. Lastly, acquired pathology such as complete stenosis of the suprarenal IVC results in anatomy similar to a congenital interrupted IVC, with development of collateral pathways and preferential blood flow into the paravertebral, azygos and hemiazygos systems (Fig. 9).
Interrupted IVC (renal level) with azygos and hemiazygos continuation. Anomaly detected incidentally on imaging for a 55-year-old man with biopsy proven renal cell carcinoma. The paired common iliac veins continue as bilateral ascending lumbar veins draining into the azygos–hemiazygos system. a Contrast-enhanced coronal CT shows the left renal vein (arrow) draining into a tortuous paravertebral collateral (asterisk). b The right renal vein (arrow) joins the suprarenal IVC (asterisk). c Axial CT image depicts a left-sided paravertebral collateral (arrow) that drains into the hemiazygos vein (asterisk) sited posteriorly. d Coronal CT image with a prominent lateral abdominal wall collateral vein
Acquired pathologies
Acquired conditions affecting the IVC include primary and secondary malignancy with or without intravascular extension, benign tumours, extrinsic compression, bland thrombus, and chronic obstruction. Primary IVC malignancy is rare, representing less than 1% of all malignancies [50, 51]. In contrast, secondary IVC malignancy is much more common and often results from tumour thrombus, i.e. direct intravascular extension from an abdominal primary such as kidney, liver or adrenal gland [16]. Metastatic lesions may also invade the caval wall, the most common of which are liver metastases from colorectal cancer [52, 53]. Lung carcinoma involving the IVC is extremely rare, and in this review, we present a case of adrenal metastases from non-small cell lung carcinoma (NSCLC) with extension into the suprarenal IVC (Fig. 11) [54]. Tumours such as pheochromocytoma and leiomyomas are typically benign; however, there are reports of IVC invasion and metastasis [55, Full size image
Extrinsic compression of IVC. Contrast-enhanced axial image demonstrating extrinsic compression of the IVC (asterisk), which is flattened, secondary to a large nodal mass (arrowhead) from lymphoma. Further lymphomatous infiltration of the right psoas and paravertebral muscles is also present (arrows)