Abstract
Background
Tuberculosis caused by Mycobacterium bovis is an important worldwide zoonosis and has been reported to cause clinical disease in several animal species, including captive wildlife. This report describes a case of M. bovis infection in a European bison from a Brazilian zoo and compiles a number of literature reports that raise concern regarding tuberculosis among captive wildlife in Brazil.
Case presentation
A 13 year-old captive-born male bison (Bison bonasus) from a Brazilian zoo began presenting weight loss, diarrhea and respiratory symptoms, which inevitably led to his death. At the animal’s necropsy, inspection of the thoracic and abdominal cavities revealed multiple enlarged lymph nodes, ranging from 4 to 10 cm, and pulmonary nodules containing caseous masses with firm white materials consistent with mineralization. Histopathology findings showed a significant amount of acid-alcohol resistant bacilli compatible with Mycobacterium spp. Specimens from lymph nodes and lungs were cultured on Petragnani and Stonebrink media, and specific PCR assays of the bacterial isolate identified it as M. bovis.
Conclusion
The European bison reported herein died from a severe form of disseminated tuberculosis caused by M. bovis. A review of the available literature indicates possible widespread occurrence of clinical disease caused by M. bovis or M. tuberculosis affecting multiple animal species in Brazilian wildlife-related institutions. These likely underestimated numbers raise concern regarding the control of the disease in captive animal populations from Brazil.
Similar content being viewed by others
Background
Tuberculosis is an important zoonosis caused by microorganisms of the Mycobacterium tuberculosis complex (MTC) and can affect human beings and several other animal species. Mycobacterium bovis and Mycobacterium tuberculosis are the most important pathogens of this complex that can cause disease in wildlife [1, 2]. Tuberculosis in zoo specimens or free-ranging populations located in national parks constitutes the majority of the reported cases of the disease in the literature [1]. Captive or movement-restrictive conditions and proximity with infected humans or livestock may contribute to the disease occurrence in these populations [3].
Over the years, many reports have detected clinical tuberculosis caused by M. bovis or M. tuberculosis in captive animals from Brazilian zoos [4–10]. Given the lack of standardized treatment protocols, these animals are frequently euthanized to avoid further spread of the disease. Besides representing a public health risk, the presence of tuberculosis in captive animals, as well as free-ranging species, represents a major negative impact on animal conservation [1]. Herein, we describe a case of severe disseminated disease caused by M. bovis in a European bison from a Brazilian zoo and compile a number of literature reports that raise concern regarding tuberculosis among captive wildlife in Brazil.
Case presentation
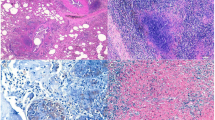
As of 2013, the Curitiba Zoological Garden, located in the Southern State of Paraná, Brazil, held 176 mammals, 480 birds, and 1101 reptiles, from 132 different species. In the second semester of that year, a 13 year-old captive-born male bison (Bison bonasus) began presenting weight loss, diarrhea, coughing and purulent nasal secretion that inevitably led to his death. At this time, the animal was kept in a wire-fenced enclosure solely with his mother. This particular enclosure is located at the border of the zoological garden, which is partly surrounded by an environmentally protected area of a river basin. At the animal’s necropsy, a poor body condition score and pale mucous membranes were noted. In the abdominal cavity, mesenteric lymph nodes were enlarged and measured from 4 to 8 cm in diameter (Fig. 1A). The iliac lymph node was 8 cm in diameter, and was causing a hydroureter as it pressed the right ureter against the abdominal wall. A hemolymph node, measuring 3.5 cm in diameter, was observed in the costal surface of the abdominal wall. The posterior ventral and right costal surfaces of the abdominal wall contained multifocal to confluent, yellow–orangish areas of consolidation, 1 cm in diameter. In the thoracic cavity, mediastinal lymph nodes (cranial, tracheobronchial, caudal) were enlarged, measuring from 7 to 10 cm in diameter. The right cranial lung lobe showed thickening of the pleura and fibrin deposition, characteristic of pleuritis, as well as small hemorrhagic foci. Multifocal to confluent, yellow–orangish areas of consolidation, from 1 to 4 cm in diameter, were observed in the lungs, predominantly on the right side (Fig. 1B). All these enlarged nodes were mostly firm and pale yellow in appearance, with a central caseous mass containing firm white material consistent with mineralization.
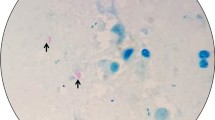
Samples from the mesenteric lymph node, pleura, and lungs were collected for histopathology. Lymph nodes and pleura sections showed similar granulomas with a high quantity of a central amorphous, basophilic material suggestive of mineralization. Lymph node tissues have been displaced against the capsule by the granulomas (Fig. 2A). Lung sections showed multifocal to confluent granulomas consisting of central necrosis surrounded by epithelioid macrophages and a few multinucleated giant cells. Lymphocytes, plasmocytes and occasionally well-differentiated fibroblasts were surrounding the granuloma (Fig. 2B). A tentative tuberculosis diagnosis was made on the basis of the clinical pathological findings and due to previous reports of MTC infecting peccaries (Tayassu tajacu) [4], a tapir (Tapirus terrestris) [6], an aoudad (Ammotragus lervia) and a waterbuck (Kobus ellipsiprymnus) (unpublished data) from the same zoo. Therefore, Ziehl–Neelsen staining was performed in a section of the lung granuloma and the presence of acid-alcohol resistant bacilli (BAAR) consistent with Mycobacterium spp (Fig. 2C) was identified.
Histopathology of tissue samples collected from a captive European bison (Bison bonasus) infected with Mycobacterium bovis. A Mesenteric lymph node showing multiple and confluent granulomatous inflammation with caseous necrosis and central mineralization pushing the lymph node tissues against its capsule. Haematoxylin and eosin stain. B Lung tissue with foci of granulomatous inflammation characterized by central necrosis surrounded by epithelioid macrophages. Lymphocytes, plasmocytes and occasionally well-differentiated fibroblasts were observed delimiting the granuloma. Haematoxylin and eosin stain. C Histochemistry of a section from the lung granuloma showing a great number of acid-alcohol resistant -alcohol resistant bacilli (BAAR) consistent with Mycobacterium spp. Ziehl–Neelsen stain
To identify the bacterial species, specimens from lymph nodes and lungs were treated by Petroff method and cultured on Petragnani and Stonebrink media at 30 and 37 °C for 90 days and inspected weekly for bacterial growth. Eugonic and dysgonic colonies suggestive of mycobacteria growth were then propagated in Stonebrink media. DNA from these cultures was extracted by a boiling method [11] and bacterial species identification was performed using PCR protocols [12, 13]. All colonies were molecularly identified as M. bovis (Fig. 3).
Gel electrophoresis of PCR products for identification of Mycobacterium bovis, using primers RD 4 [13]. Lanes 1 and 11: 100 bp ladder; 2: M. tuberculosis H37Rv, 172 bp; 3: M. bovis strain AN5, 278 bp; 4–7: DNA extracted from colonies isolated in Stonebrink media; 8 and 9: empty wells; 10: negative control (water)
Discussion
Captive European bison are exotic and rare in Brazil. Inbreeding may be a common practice to increase the number of specimens. Therefore, the bison described herein was inbred, being an offspring of a female bison with her father. Briefly, the first bison couple to be kept in the Curitiba Zoological Garden originated from another zoo located 400 km away and they were also born in captivity. They were transferred to Curitiba in the 1990s, and soon thereafter had an offspring of female twins. In 1999–2000, the father copulated with one of his daughters and generated the bison presented as a case in this report. According to the zoo archives, the first, original couple died in the early 2000s from reasons unrelated to tuberculosis. The aunt of the bison described herein died in 2008, whereas, the bison’s mother died in 2015, 2 years after him. Also between 2009 and 2010, two free-ranging coatis (Nasua nasua) from the local fauna were found dead surrounding the bison’s enclosure. Necropsy findings of the aunt, the mother, and both coatis were compatible with tuberculosis, showing granulomatous lesions in various organs and a number of acid-alcohol resistant bacilli by Ziehl–Neelsen staining. Unfortunately, tissue samples were not available for culture or molecular testing and the source of infection for all cases remains unknown.
With the current information, it is not possible to determine if the local free-ranging coati population may have served as the original infection source of this possible outbreak in the bison or vice versa. In both occasions (the aunt’s death in 2008 and the bison’s death in 2013), bison that were still in the enclosure were subjected to a preventive oral isoniazid, rifampicin and pyrazinamide treatment for eight months, even in the absence of clinical signs. Voluntary acceptance of medicated food by the animals was an issue, according to zoo records. It is also important to mention that this enclosure is isolated and does not allow contact between animals and visitors. Farm implements, uniforms and boots were exclusive of the bison’s enclosure to prevent possible disease spread within the zoo. All zookeepers and veterinarians used personal protective equipment and underwent additional tuberculin skin test and chest radiographs in each occasion (once-a-year mandatory practice in the zoo). Fortunately, zoo employees have never shown positive results for tuberculosis.
In 2001, a National Program of Control and Eradication of Bovine and Buffalo Brucellosis and Tuberculosis (PNCEBT) [14] was created in Brazil aiming to reduce the frequency of both diseases in commercial bovine herds. Wild animals, unfortunately, were not considered in the PNCEBT. Also, Brazil reports 70,000 cases and 4000 human deaths for M. tuberculosis yearly, and potential spillover of M. tuberculosis to zoo animals has been reported [6]. Since tuberculosis in wild animals is not a notifiable disease in the country, cases have been sporadically and scarcely documented. In addition to the case described herein, M. bovis or M. tuberculosis infections in wild animals have been reported in at least nine Brazilian zoos or wildlife-related institutions [4–10]. In particular, a more comprehensive study has described the isolation of M. tuberculosis or M. bovis from samples of captive llama (Lama glama), capybara (Hydrochaeris hydrochaeris), elephant (Elephas maximus), parrot (Amazona aestiva) and tufted capuchin (Cebus sp.) from four Brazilian institutions [10]. The actual extent of tuberculosis in Brazilian captive populations is therefore uncertain and likely to be underestimated.
The above-mentioned tuberculosis reports from zoos do not represent comprehensive prevalence and incidence data related to the disease in Brazilian captive wildlife. It is only possible to conclude that at least nine different zoos have reported clinical disease due to MTC in their animals over the past 8 years. Considering the negative impact tuberculosis has on public and animal health (e.g. risk of zoonotic transmission, possible euthanasia of endangered species), the authors believe that tuberculosis in zoo populations should be seriously taken into consideration when designing novel public and animal health actions related to the disease in the future.
One point to be addressed in further management plans and studies, for example, is the fact that Brazilian zoos often trade specimens and/or receive animals with unknown disease status from illegal trafficking confiscation or from other situations that preclude their reintroduction into the wild. Accurate diagnostics is pivotal to any infectious disease management. The absence of standardized diagnostic protocols for tuberculosis in several animal species may allow infected specimens to be easily introduced into captive populations, facilitating the disease spread and maintenance within and among institutions. Despite the development of tuberculosis diagnostic tests and control guidelines for elephants, non-human primates and non-domestic hoofstock [15–17], a pan-host diagnostic test is still lacking. The traditional tuberculin skin test is not applicable to all animal species and may present variable sensitivity and specificity, not identifying all truly infected animals or showing false positives due to animal exposure to environmental mycobacteria [18, 19]. Therefore, the development of sensitive and specific diagnostic tests and the establishment of specific wildlife legislation regulating animal trade, disease detection and reporting may aid in the control of tuberculosis in zoo animals.
A second point to be addressed is the identification of epidemiological factors related to tuberculosis spread inside zoos (e.g. Mycobacterium spp. environmental persistence and animal shedding routes [20, 21], aerosol transmission among different enclosures, contamination of materials and workers caring for animals, among others). Tuberculosis is a chronic disease and cases may occur over a long period of time. It is necessary to keep accurate animal records and to pursue molecular genoty** of M. bovis or M. tuberculosis isolates in each case. With such information, it is possible to detect spillovers from livestock or humans to wildlife, clonal outbreaks, and/or if genotypes brought with exotic species from abroad are being perpetuated within Brazilian zoos.
Conclusion
The European bison reported herein died from a severe form of disseminated tuberculosis caused by M. bovis. A review of the zoo archives indicates a possible outbreak involving local free-ranging coatis and other bison from the same enclosure. Also, available literature reports indicate widespread occurrence of clinical disease caused by M. bovis or M. tuberculosis affecting multiple animal species in Brazilian wildlife-related institutions. The control of the disease has been hampered by the unavailability of efficient and fast diagnostic tests and the lack of legislation regarding the control of tuberculosis in wild animal species. The occurrence of MTC in these animal populations represents a risk to animal conservation and public health in Brazil.
Abbreviations
- BAAR:
-
acid-alcohol resistant bacilli
- MTC:
-
Mycobacterium tuberculosis Complex
- PNCEBT:
-
National Program of Control and Eradication of Bovine and Buffalo Brucellosis and Tuberculosis
References
Kaneene JB, Miller R, de Kantor IN, Thoen CO. Tuberculosis in wild animals. Int J Tuberc Lung Dis. 2010;14(12):1508–12.
Hlokwe TM, van Helden P, Michel AL. Evidence of increasing intra and inter-species transmission of Mycobacterium bovis in South Africa: are we losing the battle? Prev Vet Med. 2014;115(1–2):10–7.
Rhyan JC, Spraker TR. Emergence of diseases from wildlife reservoirs. Vet Pathol. 2010;47(1):34–9.
Bonat M, Sousa R, Monego F, Javorouski M, Lacerda O, Nakatani IN, et al. Detection of Mycobacterium tuberculosis in real time PCR in a captive collared peccary (Tayassu tajacu). In: XI Congresso e XVII Encontro Associação Brasileira de Veterinários de Animais Selvagens: Anais ABRAVAS. São Paulo: Santos; 2008.
Rocha VC, Ikuta CY, Gomes MS, Quaglia F, Matushima ER, Ferreira Neto JS. Isolation of Mycobacterium tuberculosis from captive Ateles paniscus. Vector Borne Zoonotic Dis. 2011;11(5):593–4.
Murakami PS, Monego F, Ho JL, Gibson A, Javorouski ML, Bonat M, Lacerda O, et al. Detection of RDRIO Strain of Mycobacterium tuberculosis in Tapirs (Tapirus terrestris) from a Zoo in Brazil. J Zoo Wildl Med. 2012;43(4):872–5.
Murakami PS, Monego F, Ho JL, Gibson A, de Castro Vilani RG, Garcia SGC, et al. An outbreak of tuberculosis by Mycobacterium bovis in coatis (Nasua nasua). J Zoo Wildl Med. 2012;43(2):338–41.
Gomes CWC, dos Santos EO, Sonne L. Micobacteriose em arara-canindé (Ara ararauna)—relato de caso. XVI Congresso e XXII Encontro Associação Brasileira de Veterinários de Animais Selvagens: Anais ABRAVAS. Salvador; 2013.
Fredo G, Braga CS, Pietzsch CA, Wolmeister AK, Barbosa JB, Driemeier D. Linfadenite granulomatosa por Mycobacterium spp. em um macaco aranha (Ateles paniscus): achados histológicos e imuno-histoquímicos. XVIII Congresso e XXIV Encontro Associação Brasileira de Veterinários de Animais Selvagens: Anais ABRAVAS. Canela; 2015.
Ikuta CY. Estudo de micobactérias em animais silvestres mantidos em cativeiro [Study of mycobacteria in wildlife maintained in captivity]. Doctoral Thesis. Sao Paulo: University of Sao Paulo; 2015.
Fan HH, Kleven SH, Jackwood MW. Studies of intraspecies heterogeneity of Mycoplasma synoviae, M. meleagridis, and M. iowae with arbitrarily primed polymerase chain reaction. Avian Dis. 1995;39(4):766–77.
Ho H-T, Chang P-L, Hung C-C, Chang H-T. Capillary electrophoretic restriction fragment length polymorphism patterns for the mycobacterial hsp65 gene. J Clin Microbiol. 2004;42(8):3525–31.
Warren RM, van Gey Pittius NC, Barnard M, Hesseling A, Engelke E, de Kock M, et al. Differentiation of Mycobacterium tuberculosis complex by PCR amplification of genomic regions of difference. Int J Tuberc Lung Dis. 2006;10(7):818–22.
BRASIL. Programa Nacional de Controle e Erradicação da Brucelose e da Tuberculose Animal (PNCEBT). Ministério da Agricultura, Pecuária e Abastecimento. Brasília: Manual Técnico; 2006.
United States Department of Agriculture: Guidelines for the Control of Tuberculosis in Elephants. APHIS, Animal Care, Washington, District of Columbia; 2008. http://www.aphis.usda.gov/animal_welfare/downloads/elephant/elephant_tb.pdf. Accessed 10 March 2016.
National Institutes of Health: Guidelines for the Prevention and Control of Tuberculosis in Nonhuman Primates. Office of Animal Care and Use, Animal Research Advisory Committee, Bethesda; 2013. http://oacu.od.nih.gov/ARAC/documents/NHP_TB_Prevention.pdf. Accessed 02 April 2016.
United States Department of Agriculture: National Tuberculosis Surveillance Plan, Version no. 2. APHIS, Veterinary Service, Washington, District of Columbia; 2015. https://www.aphis.usda.gov/animal_health/animal_diseases/tuberculosis/downloads/tbsurveillanceplan.pdf. Accessed 03 April 2016.
Francis J, Seiler RJ, Wilkie IW, O’Boyle D, Lumsden MJ, Frost AJ. The sensitivity and specificity of various tuberculin tests using bovine PPD and other tuberculins. Vet Rec. 1978;103:420–5.
Gormley E, Doyle M, Duignan A, Good M, More SJ, Clegg TA. Identification of risk factors associated with disclosure of false positive bovine tuberculosis reactors using the gamma-interferon (IFN γ) assay. Vet Res. 2013;44(1):117.
Barasona JA, Torres MJ, Aznar J, Gortázar C, Vicente J. DNA detection reveals Mycobacterium tuberculosis complex shedding routes in its wildlife reservoir the eurasian wild boar. Transbound Emerg Dis. 2015. doi:10.1111/tbed.12458.
Barasona JA, Vicente J, Díez-Delgado I, Aznar J, Gortázar C, Torres MJ. Environmental presence of Mycobacterium tuberculosis complex in aggregation points at the wildlife/livestock interface. Transbound Emerg Dis. 2016;. doi:10.1111/tbed.12480.
Authors’ contributions
CKZ performed the necropsy, MTC diagnostics and prepared the manuscript. JSP performed the necropsy and histopathological procedures and interpretation. AWB, JHP and MB assisted with the animal necropsy. AFSF and CCD performed the cultivation and molecular identification of M. bovis. JSFN, PEB and MBH provided laboratory consumables and installation and aided in the diagnosis. AMSG coordinated the project and prepared the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank the Curitiba Zoological Garden that allowed this work to be performed. Jim Hesson of Academic English Solutions revised the manuscript (AcademicEnglishSolutions.com).
Competing interests
All authors declare that they have no competing interests.
Availability of data and materials
The findings are contained within the manuscript.
Consent for publication
The Zoo veterinarian Marcelo Bonat, co-author and responsible for this case, and the Curitiba Zoological Garden provided consent to publish this report. All measures and procedures related to disease control were the responsibility of the Curitiba Zoological Garden.
Funding
CKZ received a fellowship from the National Council for Scientific and Technological Development (CNPq), Brazilian Ministry of Science, Technology and Innovation. AMSG received a Young Talent fellowship from the Science without Borders Program, Brazilian Ministry of Education (A008_2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Cristina Kraemer Zimpel and Juliana Sperotto Brum contributed equally to this work
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zimpel, C.K., Brum, J.S., de Souza Filho, A.F. et al. Mycobacterium bovis in a European bison (Bison bonasus) raises concerns about tuberculosis in Brazilian captive wildlife populations: a case report. BMC Res Notes 10, 91 (2017). https://doi.org/10.1186/s13104-017-2413-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-017-2413-3