Abstract
Background
Bismuth salt is bacteriostatic and bactericidal against Helicobacter pylori (H. pylori). Little is known about the benefit of bismuth itself. Recently in Korea, government regulation changed to allow bismuth add-on to conventional triple eradication regimens. Study aimed the additional benefit of the bismuth add-on to the 2-week clarithromycin-based triple regimen for H. pylori eradication.
Methods
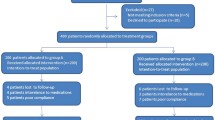
A single-centered retrospective review of electronic medical records was conducted in Seoul, Korea. Treatment-naïve H. pylori infected subjects treated with the clarithromycin-based triple regimen were consecutively enrolled. After propensity score matching, 118 subjects who were treated with rabeprazole 20 mg, amoxicillin 1 g, and clarithromycin 500 mg twice daily for 14 days (PAC) and matched 118 subjects with PAC plus bismuth subcitrate potassium 300 mg twice daily for 14 days (PACB) were included in the final analysis. The primary endpoint was the eradication success rates in each group.Article title: Kindly check and confirm the edit made in the article title.Yes, I agree with the article title.
Results
The eradication success rates were 91.5% (86.4–96.6%) for PACB regimen and 81.4% (74.2–88.5%) for PAC in the intention-to-treat analysis, and 97.3% (94.2–100%) for PACB and 88.1% (81.9–94.3%) for PAC in the per-protocol analysis. The relative risk of eradication failure for PACB over PAC was calculated as 0.184 (0.0492–0688, p value = 0.005) in multiple regression logistic analysis. Compliance and adverse event incidence were not different between the two groups.Author names: Please confirm if the author names are presented accurately and in the correct sequence (given name, middle name/initial, family name). Author 1 Given name: [Da Wit], Last name: [Shin]. Author 2 Given name: [Dae Young], Last name: [Cheung]. Author 3 Given name: [Ji Hee], Last name: [Song]. Author 4 Given name: [Fan Hee], Last name: [Lee]. Author 5 Given name: [** Il], Last name: [Kim]. Yes. I found the names presented are accurate and in the correct sequence. Author 1 Given name: [Da Wit], Last name: [Shin].Author 2 Given name: [Dae Young], Last name: [Cheung].Author 3 Given name: [Ji Hee], Last name: [Song].Author 6 Given name: [Han Hee], Last name: [Lee].Author 7 Given name: [** Il], Last name: [Kim].
Conclusion
The bismuth add-on to the 2-week clarithromycin-based triple regimen increased the eradication success rate.
Similar content being viewed by others
Introduction
Since the discovery of Helicobacter pylori (H. pylori), various antibiotics have been studied for eradication, and so far, amoxicillin and clarithromycin are recognized as having the most excellent antimicrobial effects against H. pylori [1,2,3]. For this reason, a triple combination regimen consisting of proton pump inhibitor (PPI), amoxicillin, and clarithromycin has been used as a standard treatment. However, as the frequency of resistant strains to clarithromycin increases, the eradication rate of the triple regimen gradually decreases. In Korea, clarithromycin resistance was not as high as 9% in 1995 [4], but it increased to 17.8% according to the latest study conducted by the Korean Society of Helicobacter and Upper Gastrointestinal Disease Research Group in 2018 [5]. For this reason, to overcome the decrease in the antibacterial efficacy due to the increase in clarithromycin resistance, guidelines recommends the extension of dosing period is from 7 to 14 days, or a quadruple regimen containing clarithromycin and metronidazole simultaneously, or bismuth-containing quadruple regimen as a first-line treatment [6, 7].
It is well known that bismuth has a bacteriostatic or bactericidal effect against H. pylori [21] and the authors’ study outcomes. If we cite the recent Korean research result [21] on the clarithromycin resistance, 17.8%, and eradication outcomes from Horie’s study [20], the calculated estimation of eradication success with conventional triple therapy (PAC) in Korean population is 80.3%. This simple calculation result, 80.3%, is compatible with our result of 81.4% (95% CI 0.7423–0.8849) of eradication success rate in ITT analysis for PAC group and supportive of the reliability of our study. The effect of the bismuth add-on on the clarithromycin-resistant H. pylori can also be estimated by following arithmetic calculation; [91.5% of final eradication rate in ITT analysis of PACB group = prevalence of clarithromycin-resistant H. pylori infection (17.8%) x unknown eradication rate (x) + prevalence of clarithromycin-sensitive H. pylori infection in Korea (82.2%) x unknown eradication rate (y)]. With this arithmetic calculation, the “y” value is calculated in the range of 88%(20)–100%, and “y” should not be less than “x”, then consequently the range of the “x” value should be between 52.4% and 91.5%. If the proportion of clarithromycin-resistant H. pylori increases, the lowest estimate of the interval will also increase. This is significantly higher than the 45% result of Horie et al. and is consistent with the results of other previous studies [18, 19, 22]. We can conclude that the bismuth add-on can overcome the clarithromycin resistance. Nevertheless, the authors do not advocate the use of a bismuth-added clarithromycin-based triple regimen for clarithromycin-resistant H.pylori based on the results of this study. Because it is reasonable to use metronidazole if clarithromycin resistance is known. The authors would like to emphasize that the bismuth add-on can improve the overall eradication outcomes of a 2-week clarithromycin-based triple regimen in situations where an empirical treatment is required. Contrary to the results of this study, Wu et al. reported that the eradication rate could not be improved even with a bismuth add-on for the clarithromycin-based triple regimen [23]. However, Wu et al.'s study differ from the authors' study in that it was the result of administering a clarithromycin-based triple regimen for one week in an empirical condition.
One of the concerns, when we use bismuth, is compliance and possible adverse events. In the authors’ study, the proportion of subjects who responded to take over 85% of the total doses was over 98% in both PACB and PAC groups. It looks higher than that of other reports even including prospective studies in which compliance was reported around 81–98% with the standard triple regimen [24, 25]. We admit the concern about recall bias due to the retrospective design of this study. However, the authors would like to suggest another possible explanation for better compliance than expected. All subjects in this study were prescribed two probiotics supplements along with antibiotics. Probiotics including Lactobacillus rhamnosus GG are known to help to reduce the side effects of eradication therapies and improve compliance with therapy [26]. A meta-analysis by Dang et al. reported that higher eradication rate in probiotics supplementation groups than in control (ITT analysis: RR 1.122, 95% CI 1.086–1.159, PP analysis: RR 1.114, 95% CI 1.070–1.159) and lower incidence of adverse event (RR 0.735, 95% CI 0.598–0.902) [27]. Argues are still on the table about the beneficial strains of probiotics and the definite mechanism for these effects, a variety of probiotic strains have been listed to be helpful in H. pylori eradication [28].
Regarding the adverse events, over 35% of subjects reported having discomfort or adverse events during administration. The authors acknowledge the concerns and potential for recall bias and missing records when collecting adverse event data. However, again, the authors infer that the coadministration of probiotics supplements might play a role in reducing the occurrence of possible adverse events. More importantly, the limitations of the retrospective investigation were the same for both PAC and PACB groups, and there was no difference in the incidence of adverse events according to the bismuth add-on or not. Almost all subjects in PACB group experienced dark colored stool, but the change of stool color predicted though the explanation before taking the regimen was not judged to be uncomfortable. Our results were comparable to other previous prospective studies using bismuth [25, 29] and a study from China reported 28% of adverse event incidence with 2-week PACB regimen [1]. Actually tetracycline was suggested as a major reason for adverse events in the bismuth-containing quadruple regimen[24] and bismuth itself was reported to reduce the discomfort during eradication therapy [30, 31].
Regarding rescue therapy after 1st line standard triple regimen failure, recent guidelines recommend bismuth-containing quadruple regimen as the 2nd line therapy. The authors' institution uses bismuth-containing quadruple regimen as recommended or metronidazole-based triple regimen as a 2nd line if the 1st line was clarithromycin-based triple regimen. This is most likely due to clarithromycin resistance when clarithromycin-based triple regimen therapy fails, and because metronidazole-based triple regime is generally judged to be relatively convenient to take for bismuth-containing quadruple regimen. A total of 16 of the enrolled subjects were diagnosed with treatment failure after UBT examination. The results of secondary treatment for them will be further analyzed in the future.
This study has some limitations. First, due to the retrospective study design, the concern about recall bias and missed records was inevitable. However, an important issue in attempting randomized studies to measure the effects of bismuth add-on is the ethical question of the probably expected negative effects in a control group. Recently in Korea, there have been significant changes in the usage direction of bismuth. From January 2019, under ‘Ministry of Health and Welfare Drug Notification No. 2018–174 on the standards of medical care benefits and Detailed information on the application methods’, bismuth drug can be used without restrictions to eradicate H. pylori beyond the previously approved indications. Thanks to this situational condition, the authors could carry out a parallel comparative study with and without bismuth. In this study, the two comparison groups had nearly identical baseline characteristics regarding residence area and time of intervention. The authors adopted propensity score matching analysis to minimize possible bias in outcomes by undetected factors. Second, this is a single-centered investigation. We need a multi-centered investigation to generalize our results. Third, antibiotics susceptibility test was not employed in this study. The study design was determined by considering that an empirical therapy is performed in most clinical practices, but the authors acknowledge that the interpretation of the study results would be more precise if the information on antibiotic susceptibility were available.References: As per pubmed findings, citation details [DOI] for Reference [8, 24] have been inserted. Kindly check and confirm the inserted details.Yes, I agree with the amendment.
Conclusions
The bismuth add-on to 2-week clarithromycin-based triple regimen for an empirical purpose significantly increased the eradication success rate. Bismuth add-on neither worsens the compliance nor the incidence of adverse events. Probiotics supplements during the administration of the eradication regimen possibly were helpful in reducing adverse events and improving compliance and eradication outcomes.
Availability of data and materials
Data are available upon request from the authors.
References
Lin BS, Li YY, Qiao C, Liu J, Wang J, Wan M, et al. Implementation of WeChat-based patient-doctor interaction in the management of Helicobacter pylori infection: a propensity score matching analysis. J Dig Dis. 2022;23(5–6):280–7.
Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–53.
Ford AC, Gurusamy KS, Delaney B, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive people. Cochrane Database Syst Rev. 2016;4:CD003840.
Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul. South Korea Antimicrob Agents Chemother. 2004;48(12):4843–7.
Lee JY, Kim N, Nam RH, In Choi S, Lee JW, Lee DH. Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 2019;24(6):e12660.
Jung HK, Kang SJ, Lee YC, Yang HJ, Park SY, Shin CM, et al. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver. 2021;15(2):168–95.
Kim SG, Jung HK, Lee HL, Jang JY, Lee H, Kim CG, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014;29(7):1371–86.
Yao X, **ao S, Zhou L. Integrative proteomic and metabolomic analyses reveal the mechanism by which bismuth enables Helicobacter pylori eradication. Helicobacter. 2021. https://doi.org/10.1111/hel.12846.
Chiang TH, Chen CC, Tseng PH, Liou JM, Wu MS, Shun CT, et al. Bismuth salts with versus without acid suppression for Helicobacter pylori infection: a transmission electron microscope study. Helicobacter. 2021;26(3):e12801.
Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71:1724–62.
Bang CS, Lim H, Jeong HM, Shin WG, Choi JH, Soh JS, et al. Amoxicillin or tetracycline in bismuth-containing quadruple therapy as first-line treatment for Helicobacter pylori infection. Gut Microbes. 2020;11(5):1314–23.
Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88(1):33–45.
Xu H, Wang W, Ma X, Feng R, Su Y, Cheng L, et al. Comparative efficacy and safety of high-dose dual therapy, bismuth-based quadruple therapy and non-bismuth quadruple therapies for Helicobacter pylori infection: a network meta-analysis. Eur J Gastroenterol Hepatol. 2021;33(6):775–86.
Guo B, Cao NW, Zhou HY, Chu XJ, Li BZ. Efficacy and safety of bismuth-containing quadruple treatment and concomitant treatment for first-line helicobacter pylori eradication: a systematic review and meta-analysis. Microb Pathog. 2021;152:104661.
Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5(6):321–31.
Savarino V, Vigneri S. Efficacy of 1-week ranitidine-bismuth-citrate (RBC)-based triple therapy for eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 1999;13(9):1251–2.
Chiba N. Effects of in vitro antibiotic resistance on treatment: bismuth-containing regimens. Can J Gastroenterol. 2000;14(10):885–9.
McNicholl AG, Bordin DS, Lucendo A, Fadeenko G, Fernandez MC, Voynovan I, et al. Combination of bismuth and standard triple therapy eradicates helicobacter pylori infection in more than 90% of patients. Clin Gastroenterol Hepatol. 2020;18(1):89–98.
Kim YJ, Chung WC, Kim DB. Efficacy of bismuth added to standard triple therapy as the first-line eradication regimen for Helicobacter pylori infection. Helicobacter. 2021;26(3):e12792.
Horie R, Handa O, Ando T, Ose T, Murakami T, Suzuki N, et al. Helicobacter pylori eradication therapy outcome according to clarithromycin susceptibility testing in Japan. Helicobacter. 2020;25(4):e12698.
Lee JH, Ahn JY, Choi KD, Jung HY, Kim JM, Baik GH, et al. Nationwide antibiotic resistance map** of Helicobacter pylori in Korea: a prospective multicenter study. Helicobacter. 2019;24(4):e12592.
Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65(5):870–8.
Wu MC, Wang YK, Liu CJ, Yu FJ, Kuo FC, Liu ML, et al. Adding bismuth to rabeprazole-based first-line triple therapy does not improve the eradication of helicobacter pylori. Gastroenterol Res Pract. 2017;2017:5320180.
Tian XL, Suo BJ, Zhang H, Lu HP, Li CL, Zhang YX, et al. Bismuth, esomeprazole, metronidazole and amoxicillin or tetracycline as a first-line regimen for Helicobacter pylori eradication: a randomized controlled trial. Helicobacter. 2022. https://doi.org/10.1111/hel.12935.
Kim TH, Park JM, Cheung DY, Oh JH. Comparison of 7- and 14-day eradication therapy for helicobacter pylori with first- and second-line regimen: randomized clinical trial. J Korean Med Sci. 2020;35(5):e33.
Marinelli P, Scalese G, Covelli A, Ruffa A, Bedetti G, Bruno G, et al. Lactobacillus rhamnosus GG supplementation on eradication rate and dyspepsia in Helicobacter pylori infection treated with three-in-one bismuth quadruple therapy. Front Microbiol. 2022;13:932331.
Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS ONE. 2014;9(11):e111030.
Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, et al. World gastroenterology organisation global guidelines: probiotics and prebiotics October 2011. J Clin Gastroenterol. 2012;46(6):468–81.
Lee HJ, Kim JI, Lee JS, Jun EJ, Oh JH, Cheung DY, et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015;21(1):351–9.
Ford AC, Malfertheiner P, Giguere M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J Gastroenterol. 2008;14(48):7361–70.
Sjomina O, Lielause A, Rūdule A, Vangravs R, Paršutins S, Poļaka I, et al. Randomised clinical trial: comparison of efficacy and adverse effects of a standard triple clarithromycin-containing regimen with high-dose amoxicillin and bismuth therapy in Helicobacter pylori eradication. Eur J Cancer Prev. 2022;31(4):333–8.
Funding
None.
Author information
Authors and Affiliations
Contributions
DYC designed the study. DYC and DWS conducted the data collection, analyze the data, interpretation of the results, and wrote the manuscript. JHS, KC, JL, HHL, JIK, and SP participated in the interpretation of data and manuscript reviewing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shin, D.W., Cheung, D.Y., Song, J.H. et al. The benefit of the bismuth add-on to the 2-week clarithromycin-based triple regimen for Helicobacter pylori eradication: a propensity score-matched retrospective study. Gut Pathog 15, 13 (2023). https://doi.org/10.1186/s13099-023-00539-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-023-00539-y