Abstract
Background
The use of tramadol among osteoarthritis (OA) patients has been increasing rapidly around the world, but population-based studies on its safety profile among OA patients are scarce. We sought to determine if tramadol use in OA patients is associated with increased risks of all-cause mortality, cardiovascular diseases (CVD), venous thromboembolism (VTE), and hip fractures compared with commonly prescribed nonsteroidal anti-inflammatory drugs (NSAIDs) or codeine.
Methods
Using administrative health datasets from British Columbia, Canada, we conducted a sequential propensity score-matched cohort study among all OA patients between 2005 and 2013. The tramadol cohort (i.e., tramadol initiation) was matched with four comparator cohorts (i.e., initiation of naproxen, diclofenac, cyclooxygenase-2 [Cox-2] inhibitors, or codeine). Outcomes are all-cause mortality, first-ever CVD, VTE, and hip fractures within the year after the treatment initiation. Patients were followed until they either experienced an event, left the province, or the 1-year follow-up period ended, whichever occurred first. Cox proportional hazard models were used to estimate hazard ratios after adjusting for competing risk of death.
Results
Overall, 100,358 OA patients were included (mean age: 68 years, 63% females). All-cause mortality was higher for tramadol compared to NSAIDs with rate differences (RDs/1000 person-years, 95% CI) ranging from 3.3 (0.0–6.7) to 8.1 (4.9–11.4) and hazard ratios (HRs, 95% CI) ranging from 1.2 (1.0–1.4) to 1.5 (1.3–1.8). For CVD, no differences were observed between tramadol and NSAIDs. Tramadol had a higher risk of VTE compared to diclofenac, with RD/1000 person-years (95% CI) of 2.2 (0.7–3.7) and HR (95% CI) of 1.7 (1.3–2.2). Tramadol also had a higher risk of hip fractures compared to diclofenac and Cox-2 inhibitors with RDs/1000 person-years (95% CI) of 1.9 (0.4–3.4) and 1.7 (0.2–3.3), respectively, and HRs (95% CI) of 1.6 (1.2–2.0) and 1.4 (1.1–1.9), respectively. No differences were observed between tramadol and NSAIDs for all events.
Conclusions
OA patients initiating tramadol have an increased risk of mortality, VTE, and hip fractures within 1 year compared with commonly prescribed NSAIDs, but not with codeine.
Similar content being viewed by others
Background
Osteoarthritis (OA) is the most common type of arthritis and is recognized as one of the most important health problems in modern industrial societies [1, 2]. In 2017, OA affected 303 million people globally [3]. In 2013, it was the second most costly health condition treated at United States (US) hospitals with a total of $16.5 billion in aggregate hospital costs [4]. OA is associated with cartilage degradation which can lead to pain and decreased mobility [5]. As there is no effective treatment available that can halt OA progression, the main goal of medical therapy for managing OA is to control pain while avoiding therapeutic toxicity [6]. Few safe and effective treatments are available for OA patients. Tramadol, a weak opioid agonist, has been recommended by the 2013 American Academy of Orthopaedic Surgeons guidelines and recommended conditionally by the 2012 American College of Rheumatology guidelines for symptomatic knee OA, along with nonsteroidal anti-inflammatory drugs (NSAIDs) [7, 8]. Thus, the use of tramadol among OA patients has been increasing rapidly around the world. For example, in the US, the prescription of tramadol for the management of knee OA doubled from 5 to 10% between 2003 and 2009 and 44 million tramadol prescriptions were given in 2014 [9, 10]. In the United Kingdom (UK), the prevalence of OA patients with a prescription for tramadol increased from 3 to 10% from 2000 to 2015 [11]. In the province of British Columbia (BC), Canada, tramadol use for OA patients increased steadily from its introduction in 2005 and it has been the second most commonly prescribed opioid agonist since 2008 [12].
As suggested by a recent meta-analysis on the comparative effectiveness of NSAIDs and opioid use for knee OA, there is no statistically significant difference in pain relief between tramadol and NSAIDs among OA patients [13]; however, tramadol is associated with more opioid-related adverse effects, for example, nausea, dizziness, constipation, tiredness, headache, vomiting, and drowsiness [14]. Several studies have compared risks of serious adverse events between tramadol and alternative commonly prescribed analgesics in patients with OA using the Health Improvement Network data that includes 6% of the UK population [11, 15, 16]. These studies showed that tramadol was associated with a significantly higher risk of mortality, myocardial infarction (MI), and hip fractures as compared to commonly prescribed NSAIDs. However, to describe the safety profile of tramadol among OA patients, the results need to be confirmed in a truly population-based sample. This study aimed to determine if tramadol initiation is associated with an increased risk of all-cause mortality, as well as incident cardiovascular diseases (CVD), venous thromboembolism (VTE), and hip fractures compared with other commonly prescribed analgesics for OA using the entire population of the province of BC, Canada.
Methods
Data source
Universal healthcare coverage is available for all residents of BC, Canada (population ~ 4.7 million in 2014). Population Data BC captures all provincially funded healthcare services from 1990, including all healthcare professional visits [17], hospitalizations [18], demographic data [19], BC cancer registry [20], and vital statistics [21]. Furthermore, Population Data BC includes the comprehensive prescription drug database PharmaNet [22], which captures all outpatient dispensed medications for all residents since 1996. Numerous population-based studies have been successfully conducted using Population Data BC [23,24,25,26].
Study design and cohort definitions
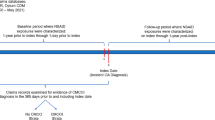
Using Population Data BC, eligible patients with OA (aged 50 years and older) who received medical care from January 1, 2005, to December 31, 2013, were included. Our case definition of OA consisted of at least two visits to a health professional within 2 years on separate days or one discharge from the hospital with an International Classification of Disease 9th revision code of 715 or International Classification of Disease 10th revision code of M15 to M19. A visit was defined as any service with the exclusion of diagnostic procedures and certain other procedures, such as dialysis/transfusion, anesthesia, obstetrics, or therapeutic radiation. Similar OA case definitions have been used in previous studies in Canada and found to have a positive predictive value varying from 82 to 100% [27, 28]. All OA patients had at least 1 year of continuous enrollment.
We conducted a sequential propensity score-matched cohort study with four comparison cohorts to assess the risk of all-cause mortality, CVD, VTE, and hip fractures between OA patients who received an initial prescription for tramadol and OA patients who received an initial prescription for one of the following medications: naproxen, diclofenac (nonselective NSAIDs), a cyclooxygenase-2 [Cox-2] inhibitor, or codeine (a commonly prescribed weak opioid) from January 1, 2005, to December 31, 2013. Eligible participants were required to have no prescriptions for tramadol or the comparator medication in the year prior to their initial prescription (i.e., the index date). Patients with a history of cancer were excluded. All participants had at least 1 year of follow-up starting from the index date.
Assessment of outcomes
Outcomes of this study were (1) all-cause mortality, (2) incident CVD (MI or ischemic stroke), (3) VTE (pulmonary embolism [PE] or deep vein thrombosis [DVT]), and (4) hip fractures within the first year following initiation of tramadol or its comparators. Case definitions for each outcome are listed in Supplemental Table 1. Similar case definitions for each outcome condition have been validated by previous studies with positive predictive values ranging between 82 and 96% [29,30,31,32].
To identify incident cases, patients with a history of each outcome event of interest prior to the index date were excluded. Patients were followed until the corresponding outcome occurred, they left the province, or the end of the 1-year follow-up period, whichever occurred first.
Statistical analysis
Calendar years from January 1, 2005, to December 31, 2013, were divided into nine 1-year blocks. Propensity scores were calculated for the initial prescription of tramadol using logistic regression. The variables included in the model were registration start date, socio-demographic factors (i.e., age at the index date and sex), OA duration, comorbidities (myocardial infarction, ischemic heart disease, heart failure, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, rheumatic disorder, chronic kidney disease, peptic ulcer disease, liver disease, diabetes, obesity, hypertension, angina, atrial fibrillation) ever prior to the index date. Comorbidities were identified using International Classification 9th and 10th revision codes. Prescriptions (aspirin, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, statins, diuretics, fibrates, nitrates, anticoagulants, antidiabetic medicines, other NSAIDs, other opioids, glucocorticoids, serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, other anti-epileptic medications) during the year prior to the index date (yes/no) were from the PharmaNet drug database. The number of healthcare utilizations was counted from the outpatient visits and hospitalizations during the year prior to the index date. We used standardized differences less than 0.10 to define a balance measure of individual covariates before and after propensity score matching [33]. Within each 1-year time block, tramadol users were matched 1:1 to the users of each of the other comparator analgesics using the greedy matching method [34]. In this way, we assembled four comparison groups: tramadol vs. naproxen, tramadol vs. diclofenac, tramadol vs. Cox-2 inhibitors, and tramadol vs. codeine.
We compared the baseline characteristics of the four tramadol cohorts with each of the four comparison cohorts both before and after the propensity score matching. We calculated person-years of follow-up for each patient and the incidence rate for each cohort and plotted cumulative incidence curves of all-cause mortality, CVD, VTE, and hip fractures. We examined the rate difference (RD)/1000 person-years in each outcome between the tramadol cohort with each of the four comparison cohorts using an additive hazard model [35]. The effect estimate generated from this model can be interpreted as the number of excess events attributable to tramadol per 1000 person-years. We compared the rate of each outcome in the tramadol cohort with each of the four comparison cohorts using Cox proportional hazard models adjusted for calendar year. We used the Fine-Gray method [36] to account for the competing risk of death for the event of CVD, VTE, and hip fractures.
All statistical analyses were performed using SAS version 9.4. For all hazard ratios (HRs), we calculated 95% confidence intervals (95% CI).
Results
As shown in Table 1, OA patients in the tramadol cohorts, in general, were older and had a longer duration of OA, a higher prevalence of comorbidities, a higher use of the majority of other prescriptions, and a higher number of healthcare visits or hospitalization than OA patients in the NSAID cohorts and the codeine cohort before propensity score matching.
After propensity score matching, 100,358 patients with OA were included (mean age of 68 years, 63% were females). Of the matched OA patients, 12,269 were included in the naproxen cohort, 15,749 in the diclofenac cohort, 15,410 in the Cox-2 inhibitor cohort, and 6751 in the codeine cohort. The baseline characteristics between the matched cohorts were well balanced, with all standardized differences less than 0.10 (Table 2).
Tramadol had a higher all-cause mortality when compared with all NSAIDs, but not with codeine (Table 3). The RDs/1000 person-years (95% CI) comparing tramadol with each comparator were 3.3 (0.0–6.7) for naproxen, 5.6 (2.3–9.0) for diclofenac, 8.1 (4.9–11.4) for Cox-2 inhibitors, and −3.8 (−9.2–1.5) for codeine. The HRs (95% CI) comparing tramadol with each comparator were 1.2 (1.0–1.4) for naproxen, 1.3 (1.1–1.5) for diclofenac, 1.5 (1.3–1.8) for Cox-2 inhibitors, and 0.9 (0.7–1.1) for codeine (Table 3, Fig. 1).
No association between tramadol and CVD (MI or ischemic stroke) was observed when compared with naproxen, diclofenac, Cox-2 inhibitors, and codeine (Table 4). The RDs/1000 person-years (95% CI) of CVD comparing tramadol with each comparator were −2.9 (−6.7–0.7) for naproxen, 0.7 (−2.6–4.0) for diclofenac, 0.5 (−2.8−3.8) for Cox-2 inhibitors, and −1.2 (−5.8–3.4) for codeine. The HRs (95% CI) of CVD comparing tramadol with each comparator were 0.9 (0.7–1.0) for naproxen, 1.0 (0.9–1.2) for diclofenac, 1.0 (0.9–1.2) for Cox-2 inhibitors, and 0.9 (0.8–1.1) for codeine (Table 4, Fig. 2). Similar results were seen in the subgroups — MI and ischemic stroke.
Tramadol had an association with VTE when compared with diclofenac (Table 5). The RDs/1000 person-years (95% CI) of VTE comparing tramadol with each comparator were 1.2 (−0.4–2.9) for naproxen, 2.2 (0.7–3.7) for diclofenac, 1.4 (−0.1–2.9) for Cox-2 inhibitors, and 1.2 (−1.2–3.7) for codeine. The HRs (95% CI) of VTE comparing tramadol with each comparator were 1.3 (1.0–1.7) for naproxen, 1.7 (1.3–2.2) for diclofenac, 1.4 (1.1–1.8) for Cox-2 inhibitors, and 1.3 (0.9–1.8) for codeine (Table 5, Fig. 3). For PE, we did not see a difference between tramadol with each outcome. For DVT, the RDs/1000 person-years (95% CI) ranged from 1.2 (0.0–2.4) to 1.5 (0.3–2.7) and HRs (95% CI) ranged from 1.5 (1.1–2.0) to 2.0 (1.4–2.8).
Tramadol had an association with hip fractures when compared with diclofenac and Cox-2 inhibitors, but not with naproxen and codeine (Table 6). The RDs/1000 person-years (95% CI) of hip fractures comparing tramadol with each comparator were 1.5 (−0.2–3.1) for naproxen, 1.9 (0.4–3.4) for diclofenac, 1.7 (0.2–3.3) for Cox-2 inhibitors, and −0.4 (−3.0–2.1) for codeine. The HRs (95% CI) of hip fractures comparing tramadol with each comparator were 1.4 (1.1–1.9) for naproxen, 1.6 (1.2–2.0) for diclofenac, 1.4 (1.1–1.8) for Cox-2 inhibitors, and 0.9 (0.7–1.3) for codeine (Table 6, Fig. 4).
Discussion
This population-based cohort study, using a large sample of people with OA from an entire Canadian province, found that tramadol initiators were at an increased risk of mortality over the following year compared with initiators of naproxen (3.3 excess deaths attributable to tramadol per 1000 person-years), diclofenac (5.6 excess deaths attributable to tramadol per 1000 person-years), and Cox-2 inhibitors (8.1 excess deaths attributable to tramadol per 1000 person-years). Tramadol initiators were also at an increased risk of DVT (1.2 to 1.5 excess DVT events attributable to tramadol per 1000 person-years) and hip fractures (1.7 to 1.9 excess hip fractures attributable to tramadol per 1000 person-years) over the following year compared with all NSAIDs except naproxen. However, tramadol initiators did not have an increased risk of CVD over the following year compared with all NSAIDs. Furthermore, no difference among all outcomes was observed between tramadol and codeine cohorts.
Both tramadol and NSAIDs are commonly used pain-relief medications for OA patients. Recently, tramadol has been considered a potential alternative to NSAIDs because of its assumed lower risk of serious cardiovascular and gastrointestinal adverse effects than NSAIDs [11]. However, despite a few recently published population-based studies [11, 15, 16], comparisons of the safety profile of tramadol with other analgesics are limited. Our study used a truly population-based sample that includes data on all healthcare encounters and dispensed medications for all persons diagnosed with OA in BC. Our results are consistent with propensity score-matched cohort studies using the general practice data in the UK [11, 15, 16]. Results from those studies showed that among patients aged 50 years and older with OA, initial prescription of tramadol was associated with a 70–100% higher risk of mortality [11] and a 65–96% higher risk of hip fractures [15] over 1-year follow-up compared with commonly prescribed NSAIDs. However, there are some discrepant results between our study and previous studies. Specifically, the UK study found that the 180-day risk of incident MI among initiators of tramadol was higher compared with naproxen [16]. They have also shown that the initiation of tramadol was associated with a higher risk of hip fractures than the initiation of codeine [15]. Another propensity score-matched cohort study using the Medicare database in the USA found that the incidence of fractures was lower in tramadol initiators than that in codeine initiators among participants with a mean age of 80 years during the 180-day follow-up period [37]. Even though our study did not demonstrate an increased risk of CVD, given that CVD risk is already increased among NSAID users [38], a non-significant difference in risk between tramadol users and NSAID users may suggest an increased risk for tramadol use as compared to those not taking either of the medications. **e et al. showed that tramadol was associated with a higher risk of all-cause mortality, compared with codeine [39]. Instead of focusing on OA patients, they investigated the association among the general population. Besides, the mean age of patients was 52.7 years in the tramadol cohort and 53.5 years in the codeine cohort, which is younger than patients in our study.
Several proposed explanations for the increased risk of mortality, VTE, and hip fractures among tramadol users compared to NSAID users exist: (1) tramadol may increase the postoperative delirium risk, which could potentially increase the risk of mortality [40]. (2) A higher risk of mortality might occur if patients take tramadol inappropriately, such as consuming alcohol or other central nervous system depressants while using tramadol [41]. (3) Tramadol might increase the coagulation of plasma proteins and inhibit the thrombocyte de-aggregation process which can increase the risk of VTE [42, 43]. (4) Tramadol may also lead to oxidative stress which has an important role in the development of atherosclerotic diseases [44, 45]. (5) Seizures [46], dizziness [47], and delirium [40] caused by tramadol could possibly increase the risk of falls which is one of the most common causes of hip fractures.
The limitations of our study deserve comment. Variables to measure OA disease severity are not available in our data. As such, confounding by indication is a potential issue in this study. Patients with more severe OA or contraindication for NSAIDs may be more likely to receive tramadol as compared to NSAIDs. While we attempted to control for confounding by indication by adjusting for propensity scores and were able to match on variables that are associated with OA pain (i.e., OA duration, comorbidities), we were unable to adjust for disease severity itself as this is not included in the administrative data. However, after the propensity score matching, all observed baseline variables were substantially balanced between comparison groups with all standardized differences less than 0.10. Second, physician-ordered dispensed medication may not reflect the actual medication use by patients and over-the-counter NSAID users may exist. Therefore, the misclassification of NSAID use could bias the results. However, all provinces in Canada have universal healthcare and PharmaNet data capture all outpatient dispensed medications for all residents. Third, the covariate OA duration may be subject to inaccuracy given that we can only capture healthcare services starting from 1990. Finally, although our sample size was large (n = 100,358), our outcomes of interest were rare, which affects the size of confidence intervals. Confidence intervals across comparison groups for a given outcome occasionally overlapped, but given the high prevalence of OA and common use of prescription medications for pain management in OA, even small differences in effect estimates are clinically important. Despite these limitations, there are several notable strengths. This is a population-based cohort study using a large Canadian administrative dataset that includes the entire population in a province and all dispensed drugs, making our results generalizable. The large sample size provided sufficient statistical power to study the safety profile of tramadol compared with commonly prescribed NSAIDs.
Conclusions
Although further evidence on the relationship between tramadol and mortality and morbidity outcomes is required, the accumulation of evidence of the risks associated with its use suggests that current guidelines on tramadol use in clinical practice might need to be revisited. As there is no difference in pain relief between tramadol and NSAIDs among OA patients [13], the potential risk of VTE and hip fractures associated with tramadol use can further increase the burden of disease in patients already afflicted with moderate to severe OA. In addition, given that risks of CVD are already increased in NSAID users, an even non-statistically significant difference of the CVD risk compared between tramadol and NSAIDs can further demonstrate an unfavorable profile of tramadol use.
In conclusion, in this population-based cohort study, we found that the initiation of tramadol was associated with a higher risk of mortality (20–50%), VTE (70%), and hip fractures (40–60%) over 1 year of follow-up compared with commonly prescribed NSAIDs, but not with codeine.
Availability of data and materials
The data that support the findings of this study are available from Population Data BC, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Change history
18 May 2022
A Correction to this paper has been published: https://doi.org/10.1186/s13075-022-02805-x
Abbreviations
- BC:
-
British Columbia
- CI:
-
Confidence interval
- Cox-2:
-
Cyclooxygenase-2
- CVD:
-
Cardiovascular diseases
- DVT:
-
Deep vein thrombosis
- HR:
-
Hazard ratio
- MI:
-
Myocardial infarction
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- PE:
-
Pulmonary embolism
- RD:
-
Rate difference
- UK:
-
United Kingdom
- US:
-
United States
- VTE:
-
Venous thromboembolism
References
Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–99.
Kopec JA, Rahman MM, Berthelot JM, Le Petit CLE, Aghajanian J, Sayre EC, et al. Descriptive epidemiology of osteoarthritis in British Columbia, Canada. J Rheumatol. 2007;34:386–93.
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013: Statistical Brief #204. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville: Agency for Healthcare Research and Quality (US); 2006.
Barnett R. Osteoarthritis. Lancet. 2018;39:1985.
Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386:376–87.
Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21:571–6.
Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465–74.
Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003-2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2014;66:1489–95.
IQVIA Institute. Medicines use and spending shifts. A review of the use of medicines in the U.S. in 2014. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/medicines-use-and-spending-shifts-in-the-us-in-2014. Published April 2015. Accessed 27 Jul 2020.
Zeng C, Dubreuil M, LaRochelle MR, Lu N, Wei J, Choi HK, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321:969–82.
Kopec J, Cibere J, Lu N, **e H, Avina-Zubieta JA, Esdaile J. Trends in prescribing of NSAIDs and opioids among osteoarthritis patients in British Columbia, Canada, 1998-2014 [abstract]. Arthritis Rheumatol. 2019;71(suppl 10) https://acrabstracts.org/abstract/trends-in-prescribing-of-nsaids-and-opioids-among-osteoarthritis-patients-in-british-columbia-canada-1998-2014/. Accessed 27 Jul 2020.
Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthr Cartil. 2016;24:962–72.
Beaulieu AD, Peloso PM, Haraoui B, Bensen W, Thomson G, Wade J, et al. Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: a randomized controlled trial. Pain Res Manag. 2008;13:103–10.
Wei J, Lane NE, Bolster MB, Dubreuil M, Zeng C, Misra D, et al. Association of tramadol use with risk of hip fracture. J Bone Miner Res. 2020;35:631–40.
Wei J, Wood MJ, Dubreuil M, Tomasson G, LaRochelle MR, Zeng C, et al. Association of tramadol with risk of myocardial infarction among patients with osteoarthritis. Osteoarthr Cartil. 2020;28:137–45.
British Columbia Ministry of Health. Medical Services Plan (MSP) Payment information file. Population Data BC. Data Extract: MOH; 2017. http://www.popdata.bc.ca/data.
Canadian Institute for Health Information. Discharge Abstract Database (Hospital Separations). Population Data BC. Data Extract: MOH; 2017. http://www.popdata.bc.ca/data.
British Columbia Ministry of Health. Consolidation File (MSP Registration & Premium Billing). Population Data BC. Data Extract: MOH; 2017. http://www.popdata.bc.ca/data
BC Cancer Registry Data. Population Data BC. Data Extract. BC Cancer; 2017. http://www.popdata.bc.ca/data.
British Columbia Ministry of Health. Vital events deaths. Population Data BC. Data Extract: MOH; 2017. http://www.popdata.bc.ca/data.
BC Ministry of Health. PharmaNet. BC Ministry of Health. Data Extract: Data Stewardship Committee; 2018. http://www.popdata.bc.ca/data.
Etminan M, Forooghian F, Brophy JM, Bird ST, Maberley D. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414–9.
Li L, McCormick N, Sayre EC, Esdaile JM, Lacaille D, **e H, et al. Trends of venous thromboembolism risk before and after diagnosis of gout: a general population-based study. Rheumatology (Oxford). 2020;59:1099–107.
Yokose C, Lu N, **e H, Li L, Zheng Y, McCormick N, et al. Heart disease and the risk of allopurinol-associated severe cutaneous adverse reactions: a general population-based cohort study. CMAJ. 2019;191:E1070–7.
Lacaille D, Avina-Zubieta JA, Sayre EC, Abrahamowicz M. Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population-closing the mortality gap. Ann Rheum Dis. 2017;76:1057–63.
Rahman MM, Cibere J, Anis AH, Goldsmith CH, Kopec JA. Risk of type 2 diabetes among osteoarthritis patients in a prospective longitudinal study. Int J Rheumatol. 2014;2014:620920.
Rahman MM, Kopec JA, Goldsmith CH, Anis AH, Cibere J. Validation of administrative osteoarthritis diagnosis using a clinical and radiological population-based cohort. Int J Rheumatol. 2016;2016:6475318.
McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: s systematic review. PLoS One. 2014;9:e92286.
McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One. 2015;10(8):e0135834.
Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S. The validity of administrative data to identify hip fractures is high--a systematic review. J Clin Epidemiol. 2013;66:278–85.
Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167:935–43.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424.
Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14:465–76.
Rod NH, Lange T, Andersen I, Marott JL, Diderichsen F. Additive interaction in survival analysis: use of the additive hazards model. Epidemiology. 2012;23:733–7.
Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–56.
Devitt N. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2011;171:1401–2.
Varga Z, Sabzwari SRA, Vargova V. Cardiovascular risk of nonsteroidal anti-inflammatory drugs: an under-recognized public health issue. Cureus. 2017;9:e1144.
**e J, Strauss VY, Martinez-Laguna D, Carbonell-Abella C, Diez-Perez A, Nogues X, et al. Association of tramadol vs codeine prescription dispensation with mortality and other adverse clinical outcomes. JAMA. 2021;326:1504–15.
Brouquet A, Cudennec T, Benoist S, Moulias S, Beauchet A, Penna C, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251:759–65.
Randall C, Crane J. Tramadol deaths in Northern Ireland: a review of cases from 1996 to 2012. J Forensic Leg Med. 2014;23:32–6.
Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, et al. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5:1199–202.
Casella S, Giannetto C, Giudice E, Marafioti S, Fazio F, Assenza A, et al. ADP-induced platelet aggregation after addition of tramadol in vitro in fed and fasted horses plasma. Res Vet Sci. 2013;94:325–30.
Ahmed MA, Kurkar A. Effects of opioid (tramadol) treatment on testicular functions in adult male rats: the role of nitric oxide and oxidative stress. Clin Exp Pharmacol Physiol. 2014;41:317–23.
Bameri B, Shaki F, Ahangar N, Ataee R, Samadi M, Mohammadi H. Evidence for the involvement of the dopaminergic system in seizure and oxidative damage induced by tramadol. Int J Toxicol. 2018;37:164–70.
Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. 2018;131:1382.e1–6.
Fricke JR, Hewitt DJ, Jordan DM, Fisher A, Rosenthal NR. A double-blind placebo-controlled comparison of tramadol/acetaminophen and tramadol in patients with postoperative dental pain. Pain. 2004;109:250–7.
Acknowledgements
All inferences, opinions, and conclusions drawn in this article are those of the authors and do not reflect the opinions or policies of the Data Steward(s). No personal identifying information was made available as part of this study.
Funding
Funding sources had no role other than funding the study. This study was funded and supported by the Canadian Institutes of Health Research (THC-135235). LL is supported by the Canadian Institutes of Health Research Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award. JAAZ is supported by the BC Lupus Society Award and is the Walter & Marilyn Booth Research Scholar.
Author information
Authors and Affiliations
Contributions
JAAZ had full access to the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read, provided critical feedback on intellectual content, and approved the final manuscript. Concept and design: JAAZ, LL, NL, and HX. Critical revision of the manuscript for important intellectual content: LL, SM, NL, HX, JAK, JC, JME, and JAAZ. Statistical analysis and data verifying: NL, LL, and HX. Obtained funding: JE, JAAZ, JK, and JC.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received ethics approval from the University of British Columbia’s Behavioural Research Ethics Board (H15-00887).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table 1
. Case definition of all-cause mortality, CVD, VTE, and hip fractures. Abbreviations: CVD, cardiovascular diseases; DVT, deep vein thrombosis; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision; MI, myocardial infarction; PE, pulmonary embolism; VTE, venous thromboembolism.
Additional file 2: Supplemental Figure 1
. Selection process of patients for the study.
Additional file 3: Supplemental Material 1
. R code for additive hazard model.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, L., Marozoff, S., Lu, N. et al. Association of tramadol with all-cause mortality, cardiovascular diseases, venous thromboembolism, and hip fractures among patients with osteoarthritis: a population-based study. Arthritis Res Ther 24, 85 (2022). https://doi.org/10.1186/s13075-022-02764-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-022-02764-3