Abstract
Background
Anopheles funestus is a major malaria vector in Eastern and Southern Africa and is currently the dominant malaria-transmitting vector in many parts of Tanzania. Previous research has identified its preference for specific aquatic habitats, especially those that persist in dry months. This observation suggests the potential for targeted control through precise habitat map** and characterization. In this study, we investigated the influence of habitat characteristics, land cover and human population densities on An. funestus distribution during dry seasons. Based on the results, we developed a habitat suitability model for this vector species in south-eastern Tanzania.
Methods
Eighteen villages in south-eastern Tanzania were surveyed during the dry season from September-December 2021. Water bodies were systematically inspected for mosquito larvae and characterized by their physico-chemical characteristics and surrounding environmental features. A generalized linear model was used to assess the presence of An. funestus larvae as a function of the physico-chemical characteristics, land use and human population densities. The results obtained from this model were used to generate spatially explicit predictions of habitat suitability in the study districts.
Results
Of the 1466 aquatic habitats surveyed, 440 were positive for An. funestus, with river streams having the highest positivity (74%; n = 322) followed by ground pools (15%; n = 67). The final model had an 83% accuracy in predicting positive An. funestus habitats, with the most important characteristics being permanent waters, clear waters with or without vegetation or movement and shading over the habitats. There was also a positive association of An. funestus presence with forested areas and a negative association with built-up areas. Human population densities had no influence on An. funestus distribution.
Conclusions
The results of this study underscore the crucial role of both the specific habitat characteristics and key environmental factors, notably land cover, in the distribution of An. funestus. In this study area, An. funestus predominantly inhabits river streams and ground pools, with a preference for clear, perennial waters with shading. The strong positive association with more pristine environments with tree covers and the negative association with built-up areas underscore the importance of ecological transitions in vector distribution and malaria transmission risk. Such spatially explicit predictions could enable more precise interventions, particularly larval source management, to accelerate malaria control.
Graphical Abstract

Similar content being viewed by others
Background
Malaria control strategies have primarily focused on insecticide-treated nets (ITNs) and indoor residual spraying (IRS) to combat mosquito vector populations. However, these interventions are currently facing major challenges, including widespread insecticide resistance and behavioural adaptations of vector species [1, 2]. In response, there is need for complementary interventions, including larval source management (LSM), which is increasingly being considered by endemic countries, particularly in urban and peri-urban settings [1, 3]. In rural areas, LSM often faces various logistical challenges, particularly due to the complexity of aquatic habitats. Existing guidelines suggest that the aquatic habitats must be 'few, fixed and findable' [4], yet in most endemic regions this is rarely the case. The sheer numbers of breeding sites as well as their dynamic nature and inaccessibility illustrate the gap between LSM guidelines and their practical implementation in diverse settings.
Successful implementation of LSM programs requires a thorough understanding of the larval ecology of the target species, and especially the ability to locate their main aquatic habitats [5]. Whether malaria transmission is seasonal or perennial, identifying the main habitats that sustain vector populations throughout the dry seasons would be particularly important since such habitats could be targeted to maximize control when the vector populations are lowest [6].
Anopheles funestus sensu stricto (Anopheles funestus s.s.) is widely recognized as a major malaria vector in Eastern and Southern Africa [7, 8]. In south-eastern Tanzania [9, 10], as well as in some districts of northern Tanzania [11], this species is now responsible for > 85% of malaria transmission. This dominance is due to several attributes of this mosquito species, including its preference for both feeding on humans indoors and resting indoors [12, 13], its strong resistance to common pyrethroid insecticides [14] and its high daily survival rates [12]. Indeed, field evidence suggests that An. funestus can dominate malaria transmission even in areas where its densities are lower than those of other malaria vector species [9]. Unfortunately, in many settings, its basic biology and ecology are less well characterized compared to those of other vector species [8].
While studies focusing on An. funestus larval ecology are scarce, some of the studies carried out so far show that whereas its aquatic habitats occasionally overlap with those of other mosquito species, An. funestus possesses certain unique attributes that underlie its preferences [15, 16]. Early studies in the 1930s provided valuable insights, indicating that An. funestus was more likely to be found in permanent water bodies, such as river streams, ditches and ponds [17, 18], unlike An. gambiae complex mosquitoes, which generally prefer smaller and less permanent habitats [19]. A more recent study in south-eastern Tanzania found that An. funestus primarily oviposits in habitats along river tributaries, and in large ponds [17]. Distinctive features of these habitats, compared to those used by other malaria vectors, included clear waters, emergent vegetation, shading, water depths exceeding 0.5 m and permanent or semi-permanent availability [17]. Given the significance of An. funestus in the region, there is a need to extend these efforts by conducting detailed analyses of the importance of land cover characteristics.
The current study was therefore designed to explore how habitat characteristics, land cover types and human population densities affect An. funestus distribution, and then to use the findings to create habitat suitability maps for the vector species in south-eastern Tanzania.
Methods
Study site
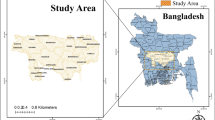
The field survey was conducted in 18 villages located in south-eastern Tanzania, including 11 villages in Ulanga district (Chikuti, Chirombora, Ebuyu, Gombe, Ikungua, Iragua, Kichangani, Kidugalo, Lukande, Mwaya and Mzelezi) and seven villages in Malinyi district (Itete, Mtimbira, Sofi Mission, Sofi Majiji, Kalengakelo, Kiswago and Ipera Asilia) in south-eastern Tanzania (Fig. 1). The area has an altitude of 250–650 m a.s.l., yearly mean temperature ranges of 20–33 °C and annual rainfall range of 1200–1800 mm [20]. Generally, the dry season occurs between June and November, short rains occur in November and December, and long rains occur from February to May [20]. The area has diverse land use features, including small towns, villages, savannahs, crops, irrigation, grazing lands, forests and shrublands. There is a large flood plain with numerous rice farms, bordered by Udzungwa mountains to the north and Mahenge hills to the south (Fig. 1). Anopheles arabiensis and An. funestus are the main malaria vectors, with the latter species mediating most of the transmission [9, 21]. The main economic activities are livestock-kee**, fishing and crop farming [22, 23].
Sampling and characterization of aquatic habitats
The habitat survey was conducted during the dry months of September to December 2021. Community members aged ≥ 18 years were recruited and trained to identify potential aquatic habitats, including natural and human-made water bodies, and to record their physico-chemical attributes, regardless of whether mosquito larvae were present or not. To ensure a comprehensive coverage of each village, a team of five people walking at a distance of 2 m from each other systematically surveyed the area along pre-set transects, within demarcations set by the village authorities.
For each water body observed during the transect walks, the team recorded: (i) time and date of visit; (ii) GPS coordinates; (iii) habitat type (classified into river streams, stagnant ground pools, marshes, wells, dug pits, brick or concrete pits, ditches, rice fields, hoofprints); (iv) habitat size (surface area); (v) water clarity; (vi) water source (rainfall accumulation or ground water); (vii) water movement (stagnant, slow or fast moving); (viii) water permanence (permanent, semi-permanent, or temporary); (ix) water depth; (x) presence and type of algal growth (brown, blue, filamentous); (xi) presence of shading; (xii) types and quantity of vegetation; and (xiii) environmental characteristics surrounding the habitats within 200 m (such as cultivation, bush areas, cattle grazing and distance to nearest human habitations). Additionally, physico-chemical metrics, including pH, total dissolved solids (TDS) and electroconductivity (EC), were measured using a water-quality meter.
Larval surveys
All identified water bodies were examined for the presence of mosquito larvae using either the standard 350-ml dipper (for small habitats with shallow depths) or a large 10-l bucket (for larger and deeper habitats), as previously described [17]. The number of dips performed in each aquatic habitat was determined based on its size, following a predefined protocol. For habitats < 5 m2, a single dip was made; in habitats measuring 6 to 10 m2, two dips were made; and for those habitats ranging in size from 11 to 15 m2, three dips were made. This incremental approach continued for larger habitats, with a limit of 20 dips for any habitat > 120 m2. Collected larvae were identified to genus and species group level, whenever possible, using standard taxonomic keys [24, 25]. Within the Anopheles genus, late instars (III and IV) of the An. funestus group and the An. gambiae complex could be easily distinguished based on their morphology [25, 26]. Consequently, in this article, the term “aquatic habitats” refers to any surveyed water body, while “positive habitats” denotes those where An. funestus was confirmed via dip**.
Environmental covariates
A digital elevation model (DEM) with 10-m resolution [27] was used to extract data on elevation, slope, terrain and aspect for each aquatic habitat location. Land cover data were derived from the European Space Agency (ESA) Sentinel-2 satellite imagery acquired in June 2022. These data consisted of eight land cover classes and had a spatial resolution of 10 m, with and an overall accuracy of 75% [28]. The ESA imagery allowed analyses of both land cover and land use characteristics, such as urban areas and forestation, and helped identify small-scale landscape features and patterns crucial for understanding the local level relationships with malaria risk [29]. For each aquatic habitat, the proportion of each land cover type (water, trees, grasslands, flooded vegetation, shrubs, built-up areas, bare ground and crops) was extracted within a 300-m buffer. In addition, the distance from each aquatic habitat to the nearest feature of each land-cover class was measured. Consideration of both the buffer zone and distance to habitats allowed for a more nuanced analysis of how both the immediate landscape composition and the proximity to specific land cover types correlate with the presence of An. funestus larvae in the aquatic habitats.
Finally, human population densities data within the 300-m buffer were obtained from the Global Human Settlement Layer (GHSL) project, a spatial raster dataset composed of 100 × 100-m cells, with each representing the number of people in that area [30].
Statistical analysis
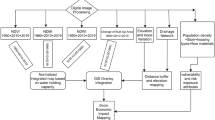
An initial descriptive analysis was conducted to assess the occurrence and distribution patterns of aquatic habitats occupied by An. funestus, as well as variations by type, specific location (village) and land cover categories (Fig. 2). A multivariate generalized linear model (GLM) with a binomial distribution was then used to examine the relationship between the presence of An. funestus larvae (absent = 0; present = 1) and a range of environmental and landscape variables (Table 1). Starting with a full model, including all the candidate variables, an automated backward stepwise selection was used to identify significant variables for inclusion in the final model, based on likelihood-ratio-tests (Table 1). To quantify the strength of association between the presence of An. funestus larvae and these variables, odds ratios (ORs) were calculated as part of the GLM. The ORs provide a measure of the likelihood of larvae presence associated with each variable.
Flowchart showing the analysis procedures, involving two datasets. On the left side, the boxes highlighted in green represent the field-collected data (dataset A), modelled using logistic regression to determine the significant variables and validated using the two-fold cross-validation technique. On the right side is a 200-m grid covering the entire study area (dataset B), which was used to perform the prediction of the habitat’s suitability based on the retained significant variables
A two-fold cross-validation process was used to validate performance of the model. The dataset was divided into a training subset (80%) and a test subset (20%). The model was trained on the training dataset and validated on the test set using Tjur's R2 calculations and area under the curve (AUC) receiver operating characteristics (ROC), with upper limits of 1.0 for a perfectly fitting model (Fig. 2).
The final model was used to generate spatial predictions of the likelihood of encountering An. funestus larvae in aquatic habitats found in different locations. To generate these maps, we created a 200-m resolution grid of regular points covering the study area of Malinyi and Ulanga districts (total area = 22,777 km2). Covariates retained in the final logistic model, such as proportions of land cover types, were extracted and applied to each grid to predict habitat suitability across the unsampled areas. To model how variations in specific habitat characteristics might influence the suitability for An. funestus, multiple scenarios were tested, with varied attribute values. For example, scenarios were created where water turbidity or habitat permanence were varied, reflecting different potential conditions.
All statistical analyses, including variable extraction, model fitting and predictions, were performed using the R statistical program version 4.2.1, with the packages rms, MASS, lme4 and glmm [31]. Preliminary data handling and visualization were performed using the software QGIS (Quantum Geographic Information System [32]).
Interactive maps for predictions and web application
To facilitate the exploration of different suitability scenarios, we also developed an interactive map using the Leaflet and Shiny packages in R [57]. Second, the accuracy of detecting mosquito larvae is influenced by sampling methods, including the number of samples, technical expertise and spatial coverage. Our study may have been limited by the pre-specified nature of these parameters. Finally, integrating field environmental data with remote sensing land cover data presents multiple challenges. For example, the resolution of land cover data used here might have been insufficient to capture fine details such as isolated residences and small water bodies, thereby impacting habitat suitability map**. To address these limitations, future studies may consider adopting higher-resolution remote sensing data sources, such as unmanned aerial vehicle (UAV) imagery [58,59,60], to capture finer details of habitats within a smaller geographical area and detect potential water sources for An. funestus [60].
Conclusions
This study comprehensively identified the main land use and environmental factors that influence larval habitat use by An. funestus during the dry season in southern Tanzania, where the species is the dominant vector of malaria transmission. We found that river streams and ground pools were the primary larval habitats during the dry season and that water bodies in forested areas, grasslands and shrublands are most likely to be positive for An. funestus. In contrast, larvae were least likely to be found in aquatic habitats which are in built-up and semi-urban areas. These insights are crucial for the strategic implementation of LSM strategies, particularly during the dry season when habitats are typically “few, fixed and findable.” The habitat suitability model developed here can be instrumental in pinpointing geographic areas where An. funestus larvae are most likely to be found, thereby facilitating targeted LSM deployment. Such targeted strategies, including larviciding and habitat modification, can be more effectively applied in high-risk zones identified through our model, thereby enhancing the efficacy of malaria control measures during the dry season. Building on these insights will further refine our understanding of mosquito dynamics, paving the way for enhanced strategies in malaria control and elimination.
Availability of data and materials
The dataset supporting findings is available upon a reasonable request directed to the corresponding author or the Ifakara health institute in Tanzania.
Change history
13 February 2024
A Correction to this paper has been published: https://doi.org/10.1186/s13071-024-06181-0
Abbreviations
- AUC:
-
Area under the curve
- DEM:
-
Digital elevation model
- EC:
-
Electroconductivity
- ESA:
-
European Space Agency
- GHSL:
-
Global Human Settlement Layer
- GUI:
-
Graphical user interface
- IRS:
-
Indoor residual spraying
- ITNs:
-
Insecticide-treated nets
- LSM:
-
Larval source management
- NDVI:
-
Normalized Difference Vegetation Index
- NDWI:
-
Normalized Difference Water Index
- QGIS:
-
Quantum Geographic Information System
- ROC:
-
Receiver operating characteristics
- TDS:
-
Total dissolved solids
- UAV:
-
Unmanned aerial vehicle
References
WHO. World malaria report 2022. 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Accessed 29 Nov 2023.
Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–91.
Hemingway J, Shretta R, Wells TN, Bell D, Djimdé AA, Achee N, et al. Tools and strategies for malaria control and elimination: what do we need to achieve a grand convergence in malaria? PLoS Biol. 2016;14:e1002380.
WHO. Larval source management: a supplementary malaria vector control measure: an operational manual. 2013. https://www.afro.who.int/publications/larval-source-management-supplementary-measure-malaria-vector-control-operational. Accessed 25 Jan 2021.
Fillinger U, Lindsay SW. Larval source management for malaria control in Africa: myths and reality. Malar J. 2011;10:1–10.
Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303.
Msugupakulya BJ, Urio NH, Jumanne M, Ngowo HS, Selvaraj P, Okumu FO, et al. Changes in contributions of different Anopheles vector species to malaria transmission in east and southern Africa from 2000 to 2022. Parasit Vectors. 2023;16:408.
Kahamba NF, Finda M, Ngowo HS, Msugupakulya BJ, Baldini F, Koekemoer LL, et al. Using ecological observations to improve malaria control in areas where Anopheles funestus is the dominant vector. Malaria J. 2022;21:1–15.
Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south–eastern Tanzania. PLoS One. 2017;12:e0177807.
Finda MF, Limwagu AJ, Ngowo HS, Matowo NS, Swai JK, Kaindoa E, et al. Dramatic decreases of malaria transmission intensities in Ifakara, south-eastern Tanzania since early 2000s. Malar J. 2018;17:1–18.
Matowo NS, Martin J, Kulkarni MA, Mosha JF, Lukole E, Isaya G, et al. An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone Tanzania. Scient Rep. 2021;11:13457.
Charlwood JD. The ecology of malaria vectors. Boca Raton: CRC Press; 2019.
Takken W, Koenraadt CJ. Ecology of parasite-vector interactions. Wageningen: Wageningen Academic Publishers; 2013.
Pinda PG, Eichenberger C, Ngowo HS, Msaky DS, Abbasi S, Kihonda J, et al. Comparative assessment of insecticide resistance phenotypes in two major malaria vectors, Anopheles funestus and Anopheles arabiensis in south-eastern Tanzania. Malar J. 2020;19:1–11.
Kweka EJ, Munga S, Himeidan Y, Githeko AK, Yan G. Assessment of mosquito larval productivity among different land use types for targeted malaria vector control in the western Kenya highlands. Parasit Vectors. 2015;8:1–8.
Debrah I, Afrane YA, Amoah LE, Ochwedo KO, Mukabana WR, Zhong D, et al. Larval ecology and bionomics of Anopheles funestus in highland and lowland sites in western Kenya. PLoS One. 2021;16:e0255321.
Nambunga IH, Ngowo HS, Mapua SA, Hape EE, Msugupakulya BJ, Msaky DS, et al. Aquatic habitats of the malaria vector Anopheles funestus in rural south-eastern Tanzania. Malar J. 2020;19:1–11.
Minakawa N, Sonye G, Dida GO, Futami K, Kaneko S. Recent reduction in the water level of Lake Victoria has created more habitats for Anopheles funestus. Malar J. 2008;7:1–6.
Mala AO, Irungu LW, Shililu JI, Muturi EJ, Mbogo CC, Njagi JK, et al. Dry season ecology of Anopheles gambiae complex mosquitoes at larval habitats in two traditionally semi-arid villages in Baringo, Kenya. Parasit Vectors. 2011;4:1–11.
WorldData.info. Climate in Morogoro, Tanzania. 2021. https://www.worlddata.info/africa/tanzania/climate-morogoro.php. Accessed 10 Oct 2021.
Mapua SA, Hape EE, Kihonda J, Bwanary H, Kifungo K, Kilalangongono M, et al. Persistently high proportions of Plasmodium-infected Anopheles funestus mosquitoes in two villages in the Kilombero valley South-Eastern Tanzania. Parasite Epidemiol Control. 2022;18:e00264.
Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS One. 2019;14:e0217414.
Swai JK, Mmbando AS, Ngowo HS, Odufuwa OG, Finda MF, Mponzi W, et al. Protecting migratory farmers in rural Tanzania using eave ribbons treated with the spatial mosquito repellent, transfluthrin. Malaria J. 2019;18:1–13.
Gillies MT, Wilkes TJ. A study of the age-composition of populations of Anopheles gambiae Giles and An. funestus Giles in North-Eastern Tanzania. Bull Entomol Res. 1965;56:237–62.
Gillies MT, De Meillon B (1968) The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). South African Institute for Medical Research: Johannesburg; 1968.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;55:1–143.
Meyer D, Tachikawa T, Kaku M, Iwasaki A, Gesch D, Oimoen M, et al. Aster global digital elevation model version 2–summary of validation results. Japan-US ASTER Science Team; 2011. p. 1–26.
Venter ZS, Barton DN, Chakraborty T, Simensen T, Singh G. Global 10 m land use land cover datasets: a comparison of Dynamic World, World Cover and ESRI Land Cover. Remote Sensing. 2022;14:4101.
Brown CF, Brumby SP, Guzder-Williams B, Birch T, Hyde SB, Mazzariello J, et al. Dynamic World, near real-time global 10 m land use land cover map**. Scientific Data. 2022;9:251.
Schiavina M, Freire S, MacManus K. GHS-POP R2022A—GHS Population Grid Multitemporal (1975–2030). 2022. European Commission, Joint Research Centre. http://data.europa.eu/89h/d6d86a90-4351-4508-99c1-cb074b022c4a. Accessed 19 Aug 2023.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing:Vienna; 2019.
QGIS Development Team AE. QGIS geographic information system. Open source geospatial foundation project. 2018. http://qgis.osgeo.org. Accessed 10 Feb 2020.
Chang, W., Cheng, J., Allaire, J., **e, Y., & McPherson, J. (2022). shiny: Web Application Framework for R. R package version 1.5. 0. 2020. Available online at http://shiny.rstudio.com/.
Hosmer Jr DW, Lemeshow S, Sturdivant RX. Applied logistic regression. John Wiley & Sons: Hoboken; 2013.
Dia I, Guelbeogo MW, Ayala D. Advances and perspectives in the study of the malaria mosquito Anopheles funestus. Anopheles mosquitoes - New Insights into Malaria Vectors, InTech, 24 July 2013. Crossref, https://doi.org/10.5772/55389.
Mwangangi JM, Mbogo CM, Muturi EJ, Nzovu JG, Githure JI, Yan G, et al. Spatial distribution and habitat characterisation of Anopheles larvae along the Kenyan coast. J Vector Borne Dis. 2007;44:44.
Omukunda E, Githeko A, Ndong’a MF, Mushinzimana E, Yan G. Effect of swamp cultivation on distribution of anopheline larval habitats in Western Kenya. J Vector Borne Dis. 2012;49:61–71.
Mendis C, Jacobsen JL, Gamage-Mendis A, Bule E, Dgedge M, Thompson R, et al. Anopheles arabiensis and An. funestus are equally important vectors of malaria in Matola coastal suburb of Maputo, southern Mozambique. Med Vet Entomol. 2000;14:171–80.
Minakawa N, Mutero CM, Githure JI, Beier JC, Yan G. Spatial distribution and habitat characterization of Anopheline mosquito larvae in Western Kenya. Am J Trop Med Hyg. 1999;61:1010–6.
Munga S, Yakob L, Mushinzimana E, Zhou G, Ouna T, Minakawa N, et al. Land use and land cover changes and spatiotemporal dynamics of anopheline larval habitats during a four-year period in a highland community of Africa. Am J Trop Med Hyg. 2009;81:1079.
Charlwood JD, Vij R, Billingsley PF. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. Am J Trop Med Hyg. 2000;62:726–32.
Lupenza ET, Kihonda J, Limwagu AJ, Ngowo HS, Sumaye RD, Lwetoijera DW. Using pastoralist community knowledge to locate and treat dry-season mosquito breeding habitats with pyriproxyfen to control Anopheles gambiae sl and Anopheles funestus sl in rural Tanzania. Parasitol Res. 2021;120:1193–202.
Silver JB. Mosquito ecology: field sampling methods. Springer Science & Business Media: Berlin/Heidelberg; 2007.
Zengenene MP, Soko W, Brooke BD, Koekemoer LL, Govere J, Mazarire TT, et al. Anopheles species composition and breeding habitat characterisation in Chiredzi District Zimbabwe. Afr Entomol. 2020;28:84–94.
Ramsdale CD, Fontaine RE, Russell E, WHO. Ecological investigations of Anopheles gambiae and Anopheles funestus. https://iris.who.int/handle/10665/65590. Accessed 15 Nov 2023.
Service, M. W. Agricultural development and arthropod-borne diseases: a review. Rev Saude Publica. 1991;25:165–78.
Kar NP, Kumar A, Singh OP, Carlton JM, Nanda N. A review of malaria transmission dynamics in forest ecosystems. Parasit Vectors. 2014;7:1–12.
Burkett-Cadena ND, Vittor AY. Deforestation and vector-borne disease: forest conversion favors important mosquito vectors of human pathogens. Basic Appl Ecol. 2018;26:101–10.
Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76:450–60.
Nicholas K, Bernard G, Bryson N, Mukabane K, Kilongosi M, Ayuya S, et al. Abundance and distribution of malaria vectors in various aquatic habitats and land use types in Kakamega County, highlands of western Kenya. Ethiop J Health Sci. 2021;31:247-56. https://doi.org/10.4314/ejhs.v31i2.7.
Ferreira CP, Godoy WA, editors. Ecological modelling applied to entomology. Vol. 1: Piracicaba. Springer: Berlin/Heidelberg; 2014. p. 219–59.
Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, Killeen GF, et al. Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar es Salaam (Tanzania) during an extended dry period. Malar J. 2005;4:1–15.
Gimnig JE, Ombok M, Kamau L, Hawley WA. Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. J Med Entomol. 2001;38:282–8.
Paaijmans KP, Jacobs AFG, Takken W, Heusinkveld BG, Githeko AK, Dicke M, et al. Observations and model estimates of diurnal water temperature dynamics in mosquito breeding sites in western Kenya. Hydrol Proc Int J. 2008;22:4789–801.
Smith MW, Willis T, Alfieri L, James WHM, Trigg MA, Yamazaki D, et al. Incorporating hydrology into climate suitability models changes projections of malaria transmission in Africa. Nat Commun. 2020;11:4353.
Hardy AJ, Gamarra JG, Cross DE, Macklin MG, Smith MW, Kihonda J, et al. Habitat hydrology and geomorphology control the distribution of malaria vector larvae in rural Africa. PLoS One. 2013;8:e81931.
Hardy A, Mageni Z, Dongus S, Killeen G, Macklin MG, Majambare S, et al. Map** hotspots of malaria transmission from pre-existing hydrology, geology, and geomorphology data in the pre-elimination context of Zanzibar, United Republic of Tanzania. Parasit Vectors. 2015;8:1–15.
Byrne I, Aure W, Manin BO, Vythilingam I, Ferguson HM, Drakeley CJ, et al. Environmental and spatial risk factors for the larval habitats of Plasmodium knowlesi vectors in Sabah Malaysian Borneo. Sci Rep. 2021;11:11810.
Minakshi M, Bhuiyan T, Kariev S, Kaddumukasa M, Loum D, Stanley NB, et al. High-accuracy detection of malaria mosquito habitats using drone-based multispectral imagery and Artificial Intelligence (AI) algorithms in an agro-village peri-urban pastureland intervention site (Akonyibedo) in Unyama SubCounty, Gulu District, Northern Uganda. J Public Health Epidemiol. 2020;12:202–17.
Hardy A, Makame M, Cross D, Majambere S, Msellem M. Using low-cost drones to map malaria vector habitats. Parasit Vectors. 2017;10:1–13.
Acknowledgements
We express our gratitude to the community leaders and residents in all the study villages for granting us permission to carry out our work in their localities. We also extend our thanks to the technicians and volunteers, including Amos Ngonzi, Kareem Mchembo, Richard Lumuli and others, who actively contributed to the successful execution of this comprehensive survey.
Funding
This work was supported in whole by the Bill & Melinda Gates Foundation (Grant No. INV-002138 to Ifakara Health Institute). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Author information
Authors and Affiliations
Contributions
NFK, FOO and HMF conceptualized the study and designed the research framework. NFK, KK and MJ were involved in data collection. NFK took the lead in producing the initial and subsequent drafts of the manuscript. HMF critically reviewed the initial drafts of the manuscript. HMF and FOO provided guidance throughout the research process and also provided insights during data analysis and interpretation. LN provided expertise and guidance in the study's statistical analysis, and reviewed and interpreted the statistical results. LN and FOO proofread the final draft of the manuscript. HMF, FOO, LN, FB, MJ and JO critically reviewed and revised the manuscript. All authors have read and approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Ethical considerations and consent to participate
Ethical approvals for this project were obtained from Ifakara Health Institute’s Institutional Review Board (Protocol ID: IHI/IRB/No: 26-2020) and the Medical Research Coordinating Committee (MRCC) at the National Institute for Medical Research (Protocol ID: NIMR/HQ/R.8a/Vol.IX/3495).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: Luca Nelli's name was misspelled Luca Neill.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kahamba, N.F., Okumu, F.O., Jumanne, M. et al. Geospatial modelling of dry season habitats of the malaria vector, Anopheles funestus, in south-eastern Tanzania. Parasites Vectors 17, 38 (2024). https://doi.org/10.1186/s13071-024-06119-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06119-6