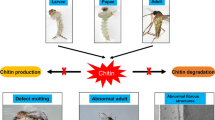
Abstract
Background
Mosquitoes are an important vector of viral transmission, and due to the complexity of the pathogens they transmit, vector control may be the most effective strategy to control mosquito-borne diseases. Chitin is required for insect growth and development and is absent in higher animals and plants, so regulating the chitin synthesis pathway can serve as a potentially effective means to control vector insects. Most of the current research on the chitin synthase (CHS) gene is focused on chitin synthase-1 (CHS-1), while relatively little is known about chitin synthase-2 (CHS-2).
Results
The CHS-2 gene of Ae. albopictus is highly conserved and closely related to that of Aedes aegypti. The expression of CHS-2 in the third-instar larvae and pupal stage of Ae. albopictus was relatively high, and CHS-2 expression in adult mosquitoes reached the highest value 24 h after blood-feeding. In the fourth-instar larvae of Ae. albopictus, CHS-2 expression was significantly higher in the midgut than in the epidermis. Silencing CHS-2 in Ae. albopictus larvae had no effect on larval survival and emergence. The expression of four genes related to chitin synthesis enzymes was significantly upregulated, the expression level of three genes was unchanged, and only the expression level of GFAT was significantly downregulated. The expression of chitin metabolism-related genes was also upregulated after silencing. The level of chitin in the midgut of Ae. albopictus larvae was significantly decreased, while the chitinase activity was unchanged. The epithelium of the midgut showed vacuolization, cell invagination and partial cell rupture, and the structure of the peritrophic membrane was destroyed or even absent.
Methods
The expression of CHS-2 in different developmental stages and tissues of Aedes albopictus was detected by real-time fluorescence quantitative PCR (qPCR). After silencing CHS-2 of the fourth-instar larvae of Ae. albopictus by RNA interference (RNAi), the expression levels of genes related to chitin metabolism, chitin content and chitinase activity in the larvae were detected. The structure of peritrophic membrane in the midgut of the fourth-instar larvae after silencing was observed by paraffin section and hematoxylin–eosin (HE) staining.
Conclusion
CHS-2 can affect midgut chitin synthesis and breakdown by regulating chitin metabolic pathway-related genes and is involved in the formation of the midgut peritrophic membrane in Ae. albopictus, playing an important role in growth and development. It may be a potential target for enhancing other control methods.
Graphical Abstract

Similar content being viewed by others
Background
Mosquitoes feed on the blood of various animals and humans and transmit viruses that cause many diseases, such as dengue, malaria, chikungunya, and yellow fever. Several species of mosquitoes have a strong invasive capacity [1]. At present, mosquito-borne diseases are a great global public health challenge [2, 3]. Due to the complexity of mosquito-borne pathogens, eradication of vectors has become one of the more effective strategies for controlling infectious diseases. At present, the main method for mosquito control is the use of chemical insecticides, such as pyrethroids [4]. However, these insecticides can affect non-target organisms [5], and it has been shown that mosquitoes have already developed resistance to chemical insecticides [6, 7]. Therefore, there is an urgent need to find new eco-friendly insecticidal methods. Currently, the alternatives mainly include plant-derived insecticides [8, 9], genetically modified mosquitoes [10,11,12], and insecticidal microorganisms [13, 14].
Chitin is a linear polysaccharide polymer composed of multiple N-acetylglucosamines connected by β-1,4 glycosidic bonds. It is currently the second-largest known biopolymerized polysaccharide in the world, second only to cellulose [15]. It is present in the shell of invertebrates and the cell wall of fungi, and mainly plays a role in support, defence, and water loss prevention [16]. In insects, chitin is found mainly on the epidermis, trachea, and peritrophic membrane [17]. The midgut is the main digestive organ of insects, and it is also the entry point for a variety of pathogens in addition to its central role in the digestion and absorption of nutrients. The peritrophic membrane is a noncellular semipermeable structure surrounding the midgut that can protect the midgut from mechanical damage and can also block the entry of microbial pathogens and macromolecular aggregates. It is the first line of defence in the insect midgut [18]. However, in pest control efforts, it is also an obstacle for the entry of insecticides and insecticidal microorganisms [19]. Therefore, how to destroy the peritrophic membrane and make the insecticidal substances more effective has become a research focus [20,21,22]. The main components of the peritrophic membrane are protein and chitin. Previous studies have shown that chitin plays an indispensable role in the barrier of the peritrophic membrane [23]. Moreover, mammals and plants do not contain chitin [24], so the synthesis of chitin and the formation of peritrophic membranes have become potential targets for pest control. Specific control of chitin synthesis and degradation has become a new approach for pest control [25, 26], in view of the effect of simple use of inhibitors of chitin synthesis such as benzophenylurea (BPU) or other chemical compounds on non-target organisms such as bees [27].
The synthesis of chitin is an extremely complex process. Existing studies have shown that the raw material for chitin synthesis in insects is trehalose [28], which transforms into chitin through the action of trehalase (TRE), hexokinase (HK), glucose-6-phosphate isomerase (G6PI), glutamine–fructose-6-phosphate aminotransferase (GFAT), glucosamine-6-phosphate-n-acetyltransferase (GNPNA), phosphoacetylglucosamine mutase (PAGM), uridine diphosphate (UDP)-N-acetylglucosamine pyrophosphorylase (UAP), and chitin synthetase (CHS) [29].
The most important enzymes in the chitin synthesis pathway are TRE at the initial step and CHS at the final step [30]. CHS is a member of the glycosyltransferase family and is a large transmembrane protein with a molecular weight of 160–180 kD [31]. A comparison of CHS in various insects showed that CHS contained three conserved domains: domains A, B, and C. Domain A is located at the N end and is a transmembrane helix, domain B is the active centre of CHS, and domain C is located in terminal C and contains seven transmembrane helices [32]. Most insects contain two kinds of CHS, chitin synthetase A (CHSA) and chitin synthetase B (CHSB), which are encoded by CHS-1 and CHS-2, respectively [33]. Several studies have shown that the two genes are involved in the synthesis of chitin in different insect body parts. CHS-1 is mainly involved in chitin synthesis in the epidermis and trachea, while CHS-2 is highly expressed only in insect midgut epithelial cells and is responsible for synthesizing chitin in the peritrophic membrane [34]. Since Tellam et al. first cloned the full-length complementary DNA (cDNA) sequence of the CHS gene of Lucilia cuprina [32], the CHS sequences of Drosophila melanogaster [35], Tribolium castaneum [36], Spodoptera exigua [37], Anopheles gambiae [38], Leptinotarsa decemlineata [39], and other insects have been cloned. There have been several studies on CHS as a potential target for pest control [40, 41], but these studies mainly focused on CHS-1; for example, after CHS-1 gene expression levels were decreased in Culex pipiens pallens larvae and pupae, the moulting, pupation, and eclosion of the larvae were affected [42]. Guo et al. confirmed that CHS-1 was involved in the development of drug resistance in Cx. pipiens pallens [43]. Huang et al. investigated interference with the normal expression of CHS-1 in Diaphorina citri by RNA interference (RNAi), which caused the mortality and deformity rate to increase significantly and the moulting rate to decrease [44]. Zhang et al. demonstrated that after interference with Locusta migratoria CHS-1 and its two variants, migratory locusts showed increased mortality and moulting rates [45]. Mohammed et al. studied knockdown of the CHS-1 gene of Phthorimaea operculella, and larvae exhibited difficulty moulting and increased mortality [46].
Aedes albopictus, also known as the Asian tiger mosquito, belongs to the order Diptera, and is an important viral vector that can spread many pathogens such as dengue fever and yellow fever [47]. Aedes albopictus has a strong reproductive ability, and its eggs have strong drought resistance and vitality, can be stored for a long time in a dry environment, and can be easily carried and spread [48].
In this study, we analysed the expression of CHS-2 in Ae. albopictus, used RNAi technology to silence the CHS-2 gene in Ae. albopictus, and combined histological observation and biochemical analysis to study the function of the CHS-2 gene at the messenger RNA (mRNA) level and enzyme activity level and explore the possibility of CHS-2 as a potential target for controlling the Ae. albopictus population.
Methods
Mosquitos
Aedes albopictus were provided by Sun Yat-sen University (Guangdong Province, China), and were fed and stably passaged for more than three generations in an artificial climate chamber at 27 ± 1 °C with 70% relative humidity and light/dark (L:D) = 14:10. Cat food and yeast powder were ground into a mixed powder at a 2:1 ratio for feeding first- and second-instar larvae. The third- and fourth-instar larvae were fed cat chow powder, and post-eclosion adults were fed 10% sugar water. Mice were used as blood samples for female mosquitoes to induce egg-laying, following the method of Hsu et al. [49].
Cloning and analysis of CHS-2 in Ae. albopictus
Total RNA was extracted from the fourth-instar larvae of Ae. albopictus using the TRIzol method. The quality and concentration of RNA were detected by agarose gel electrophoresis and microspectrophotometry (Thermo Fisher, USA). cDNA was synthesized according to the instructions of the Prime Script™ RT Reagent Kit (Haofeng, Hangzhou, China). The sequence of the CHS-2 gene (LOC109405983) of Ae. albopictus was found using the National Center for Biotechnology Information (NCBI) database, and specific primers (Table 1) were designed using Primer Premier 5.0 software to amplify intermediate sequence fragments with the cDNA template obtained in the polymerase chain reaction (PCR) system. The reaction mixture included cDNA (1 μl), forward and reverse primers (1 μl), dNTP (2.5 mM, 2 μl), 10× buffer (Mg2+, 2.5 μl), 5 U/μl Ex Taq (0.2 μl), and double-distilled water (ddH2O) (17.3 μl). PCR was performed using the following conditions: 5 min at 95 °C; 30 s at 95 °C, 30 s at 58 °C, and 1 min at 72 °C, for a total of 30 cycles, and then 10 min at 72 °C. The PCR products were separated and identified by electrophoresis on a 1% agarose gel, and the target bands were recovered by a DiaSpin PCR Product Purification Kit (Sangon Biotech, Shanghai, China), ligated into the pMD™ 18-T vector (Haofeng, Hangzhou, China), and transformed into Escherichia coli DH5α. After ampicillin screening, positive clones were selected and sequenced after screening with ampicillin (Shangya, Hangzhou, China). Phylogenetic trees were constructed using the neighbor-joining method in MEGA 5.0, with Jones–Taylor–Thornton as the model. Evolutionary distances were calculated by Poisson correction [50].
Determination of spatio-temporal expression patterns of the CHS-2 gene in Ae. albopictus
The normally raised Ae. albopictus eggs (12 h), third- and fourth-instar larvae, pupae, adult Ae. albopictus (female) at 24 h and 48 h after eclosion, and adult Ae. albopictus (female) at 24 h and 48 h after blood-sucking were collected. At the same time, the midgut and epidermis of the fourth-instar larvae were collected by dissection in 1× phosphate-buffered solution (PBS). Larvae and tissues from 300 mg were collected from each group for the experiments. RNA was extracted, and cDNA was synthesized for real-time fluorescence-based quantitative PCR (qPCR). The qPCR reaction mixture (10 µl) contained 5 µl of TB Green® Premix Ex Taq™, 3.2 µl of ddH2O, and 0.4 µl each of upstream and downstream primers and 1 µl cDNA. The qPCR procedure was as follows: 10 min at 94 °C; 30 s at 94 °C, 30 s at 59 °C, 45 s at 72 °C for a total of 30 cycles, and then 10 min at 72 °C. The default dissolution curve analysis was used. The actin gene (NCBI: DQ657949.1) was used as an internal control [51], and the 2−△△CT method was used to calculate the relative expression of CHS-2 in the tissues of Ae. albopictus at each developmental stage and fourth-instar larvae.
Microinjection
The successfully sequenced CHS-2 fragment was used as a template, and T7 promoter sequences were added to the 5′ end of CHS-2 primers (Table 2). Double-stranded RNA (dsRNA) was synthesized using a T7 RiboMAX™ Express RNAi System kit (LABOOT, Hangzhou, China) and purified. The same method was used to synthesize dsRNA) of the green fluorescent protein (GFP) gene as a negative control. Well-developed Ae. albopictus fourth-instar larvae of the same size were randomly selected. Two hundred nanograms of dsCHS-2 was dissolved in 100 nl of water and injected into the soft spot between the penultimate and third-to-last body segments of larvae using a microinjection device, and the same amount of dsGFP was injected as a negative control. Three biological replicates were set up, and 150 larvae were injected in each replicate. The injected larvae were reared normally. Larval mortality was recorded at 24 h and 48 h after injection, and the rates of pupation and emergence of these pupae were recorded within 72 h after injection. Fluorescence qPCR was used to detect the relative expression of the CHS-2 gene in larvae at 24 h and 48 h after injection. The reaction mixture was the same as above.
Evaluation of chitin synthesis-related genes
Ten fourth-instar larvae were randomly selected 24 h and 48 h after injection of dsCHS-2 and dsGFP. RNA was extracted and cDNA was synthesized. Through NCBI and related literature, the genes related to chitin synthesis and chitin metabolizing enzymes were selected, including TRE1 (NCBI: XM_029860981.1), TRE2 (NCBI: XM_0196760980), HK (NCBI: AY705876.1), G6PI (NCBI: XM_019673634.3), GFAT (NCBI: XM_019671518.2), GNPNA (NCBI: XM_019707567.2), PAGM (NCBI: XM_019671844.2), UAP (NCBI: GAPW01001510.1), Cht2 (NCBI: XM_029879282.1), and Cht10 (NCBI: XM_029869372.1). Primer Premier 5.0 software was used to design qPCR primers (Table 3), and the expression level was measured by qPCR. The reaction system and procedure were the same as above. Fourth-instar larvae 24 h after injection of dsCHS-2 and dsGFP were selected again and dissected. The level of chitin in the midgut of larvae was determined according to the method reported by Liu et al. [52], and the principle is that chitinase hydrolyses chitin to produce N-acetylglucosamine, and the intermediate compound produced by the cross-reaction of N-acetylglucosamine and alkali can further react with p-dimethylaminobenzaldehyde to produce chromogenic substances. Chitinase activity in the midgut of Ae. albopictus larvae was evaluated using a chitinase kit (Jiancheng, Nan**g, China). The midgut protein concentration was measured using a BCA protein concentration assay kit (Takara, Japan), and the specific experimental steps were in accordance with the instructions. Tissues were collected from 45 larvae in each group for the experiments.
Observation of the peritrophic membrane of the midgut
Fourth-instar larvae 24 h and 48 h after injection of dsRNA were used as materials, and the head and tail were removed according to the Gregor et al. production, sectioning, and staining method [53], fixed with formaldehyde, washed with gradient concentrations of ethanol, and then washed with xylene to make them transparent. The larvae were embedded in paraffin with an embedding machine (MICROM, Germany) and sectioned into 5-µm-thick sections. After deparaffinization with xylene, the samples were cleaned with gradient concentrations of ethanol and double-distilled water. The samples were stained with hematoxylin–eosin (HE) staining. After adding coverslips to the slides, a microscope camera system (Axio Observer A1 + Stemi2000, ZEISS, Germany) was used to photograph and observe.
Data analysis
Excel was used to organize the data, and IBM SPSS Statistics 23.0 and one-way analysis of variance (ANOVA) with Duncan’s test were used for significance analysis (significant differences indicated as *P < 0.05, **P < 0.01). SigmaPlot 10.0 was used for the plot. All experiments were set up with three biological replicates and three technical replicates.
Results
Cloning and sequence analysis of the CHS-2 gene of Ae. albopictus
The full-length CHS-2 gene sequence of Ae. albopictus (NCBI: XM_019679038.3) was obtained by searching the NCBI database. The full length of the CHS-2 gene was 6206 base pairs (bp), and we amplified a 463-bp-long fragment of the gene starting at 766 bp and ending at 1229 bp for dsRNA synthesis. An evolutionary tree was constructed by selecting the CHS-2 amino acid sequences of 20 species of insects with similar homology to Ae. albopictus, including Aedes aegypti, L. migratoria manilensis, Culex quinquefasciatus, D. melanogaster, Tribolium subtilis, and Diprionidae, which are relatively close homologues to Ae. albopictus (Fig. 1).
Spatio-temporal expression pattern of Ae. albopictus CHS-2 gene
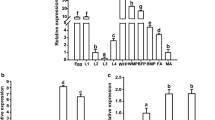
The expression of Ae. albopictus CHS-2 in the adult mosquitoes at 24 h after blood-sucking reached the highest value, followed by the third-instar larvae and pupal stages. After eclosion, the expression level of CHS-2 in adult mosquitoes at 24 h and 48 h gradually decreased; the expression in adult mosquitoes at 48 h after blood-sucking was significantly decreased, and was almost at the same level as that in the initial egg stage (Fig. 2A). The expression of the CHS-2 gene in the midgut was significantly higher than that in the epidermis in fourth-instar larvae (ANOVA, F (1, 4) = 225.941, P = 0.004) (Fig. 2B).
Expression patterns of CHS-2 gene in Ae. albopictus. Real-time quantitative fluorescence PCR (qPCR) was used to detect the relative expression level of CHS-2 in different periods of Ae. albopictus and in different tissues of the fourth-instar larvae of Ae. albopictus. A Relative expression of CHS-2 in Ae. albopictus at different stages: egg, third-instar larva (3rd); fourth-instar larva (4th); adult Ae. albopictus (female) at 24 h after eclosion (AD1); adult Ae. albopictus (female) at 48 h after eclosion (AD2); adult Ae. albopictus (female) at 24 h after blood-sucking (AD3); adult Ae. albopictus (female) at 48 h after blood-sucking (AD4). Different lowercase letters above the bar indicate that the difference is statistically significant (one-way ANOVA, Tukey test, P < 0.05). B Relative expression of CHS-2 in the midgut and cuticle of fourth-instar larvae. Relative expression levels were calculated in comparison with that at 4th (A) and cuticle (B), which were ascribed an arbitrary value of 1. Three biological replicates and three technical replicates were set. (One-way ANOVA, Duncan test, *P < 0.05, **P < 0.01)
Evaluation of the silencing effect of CHS-2
After Ae. albopictus was injected with dsRNA, the expression of the CHS-2 gene at 24 h after silencing was significantly downregulated compared to that in the control group (ANOVA, F (1, 4) = 153.813, P = 0.006), and the expression at 48 h after silencing was also significantly downregulated (ANOVA, F (1, 4) = 38.103, P = 0.025) (Fig. 3), indicating that the effect of RNA silencing was obvious, and the follow-up experiments could be carried out. CHS-2 gene silencing had no effect on the survival of Ae. albopictus larvae (Fig. 4A). Pupation and emergence of larvae within 72 h after silencing were also unaffected (Fig. 4B).
Detection of silencing efficiency. The relative expression of the CHS-2 gene in Ae. albopictus larvae was detected by real-time quantitative PCR (qPCR) 24 h and 48 h after dsRNA injection. Relative expression levels were calculated in comparison with those at 24 h after dsGFP injection, which was ascribed an arbitrary value of 1. Three biological replicates and three technical replicates were set. (One-way ANOVA, Duncan test, *P < 0.05, **P < 0.01)
Survival and development of fourth-instar larvae of Ae. albopictus after CHS-2 gene silencing. Fifty larvae of the fourth-instar Ae. albopictus were randomly selected and fed normally after injection of dsRNA. The mortality rate of larvae after 24 h and 48 h (A) and the pupation rate and emergence rate of larvae within 72 h (B) were recorded. Three biological replicates and three technical replicates were set. (One-way ANOVA, Duncan test)
Changes in the expression of genes involved in the chitin synthesis pathway after CHS-2 inhibition
Compared with the control group, the dsCHS-2 treatment group exhibited significant upregulation of the chitin synthesis-related gene TRE1 in Ae. albopictus (ANOVA, F (1, 4) = 42.348, P = 0.003), and the recovery at 48 h was consistent with the control group (Fig. 5A). TRE2 (ANOVA, F (1, 4) = 66.4, P = 0.04) and chitin metabolism-related Cht2 (ANOVA, F (1, 4) = 90.11, P = 0.002) and Cht10 (ANOVA, F (1, 4) = 51.343, P = 0.019) were significantly upregulated at 24 h and 48 h (ANOVA, TRE2 F (1, 4) = 92.384, P = 0.001, Cht2 F (1, 4) = 8.294, P = 0.045, Cht10 F (1, 4) = 112.28, P = 0.002) after injection (Fig. 5A, B). In addition, HK (ANOVA, F (1, 4) = 8.414, P = 0.044) and G6PI (ANOVA, F (1, 4) = 11.26, P = 0.044) gene expression were significantly upregulated after CHS-2 silencing (Fig. 5C, G). Among the remaining genes, PAGM, UAP, and GNPNA showed no significant change (Fig. 5D, E, H); only GFAT (ANOVA, F (1, 4) = 29.025, P = 0.013) expression was significantly downregulated 24 h after silencing (Fig. 5F).
Expression of genes involved in chitin synthesis pathway in Ae. albopictus larvae after CHS-2 silencing. A TRE1 and TRE2. B Cht2 and Cht10. C HK. D PAGM. E GNPNA. F GFAT. G G6PI. H UAP. Relative expression levels were calculated in comparison with those after dsGFP injection, which was assigned an arbitrary value of 1. Three biological replicates and three technical replicates were set. (One-way ANOVA, Duncan test, *P < 0.05, **P < 0.01)
Changes in chitin levels and enzyme activity after CHS-2 inhibition
At 24 h after injection of dsCHS-2, the chitin level in the midgut of Ae. albopictus was significantly reduced compared with the control (ANOVA, F (1, 4) = 21.653, P = 0.043) (Fig. 6A). However, after inhibiting CHS-2 for 24 h, there was no significant change in chitinase activity in the midgut of the fourth-instar larvae of Ae. albopictus (Fig. 6B).
Changes of chitin content and enzyme activity in the midgut of Ae. albopictus larvae after CHS-2 silencing. The fourth-instar larvae were dissected and the midguts of 45 larvae were collected at 24 h and 48 h after the injection of dsRNA. The content of chitin in the midgut of the larvae was determined by chitinase, and the activity of chitinase in the midgut of the larvae was determined by a chitinase kit. A Chitin content in the midgut of the fourth-instar larvae of Ae. albopictus 24 h after dsRNA injection. B Chitinase activity in the midgut of the fourth-instar larvae of Ae. albopictus 24 h after dsRNA injection. Three biological replicates and three technical replicates were set. (One-way ANOVA, Duncan test, *P < 0.05, **P < 0.01)
Morphological changes in the midgut after CHS-2 inhibition
At 24 h and 48 h after injection of dsCHS-2, the midgut epithelial cells of Ae. albopictus larvae showed vacuolization, intracellular convexity, and partial cell rupture, and the structure of the peritrophic membrane was destroyed or even absent. In the control group injected with dsGFP, the peritrophic membrane of the midgut was fully developed (Fig. 7).
Section of fourth-instar larvae of Ae. albopictus after CHS-2 silencing. Fourth-instar larvae 24 h and 48 h after injection of dsRNA were used as materials; the head and tail were removed, the larvae were embedded in paraffin, sectioned, and stained with HE, and they were observed with a microscope: A 24 h after the injection of dsGFP, B 48 h after the injection of dsGFP, C 24 h after the injection of dsCHS-2, D 48 h after the injection of dsCHS-2. Scale indicates 100 μm, the black arrow in the figure is the normal peritrophic membrane
Discussion
Chitin synthase, the most important enzyme of the chitin synthesis pathway in insects, is a conserved enzyme present in all chitin-containing organisms. Tellam et al. first identified the gene sequence encoding insect chitin synthase in 2000 [54]. Since then, with the development of gene, protein, and transcriptome technologies, more genes that encode insect chitin synthetases have been discovered [70]. In this study, the CHS-2 gene of Ae. albopictus was silenced by RNAi, the relative expression level was significantly downregulated, and the inhibitory effect was obvious (Fig. 3). CHS-2 silencing had no significant influence on the survival of larvae and mature larvae (Fig. 4); the reason may be that the short duration of dsRNA action in microinjection or the possible impact of mechanical damage in microinjection on larval survival.
The expression of genes related to the chitin synthesis pathway was also measured. TRE is the first enzyme in the chitin synthesis pathway, and silencing TRE resulted in decreased expression levels of genes involved in chitin metabolism [71]. After knockdown of CHS-2 for 24 h and 48 h, the expression of TRE2 was significantly upregulated (Fig. 5A). Twenty-four hours after silencing, TRE1 expression was significantly upregulated (Fig. 5A). HK and G6PI are also important enzymes in the chitin synthesis pathway [72, 73], and their expression levels were also significantly upregulated after CHS-2 silencing (Fig. 5C, G). The above are all genes upstream of CHS-2 in the chitin synthesis pathway, and after CHS-2 is inhibited, these genes may be highly expressed in the short term to synthesize more chitin from other sites in response to the loss of chitin in the midgut. However, the expression of GFAT was significantly downregulated and returned to the same level as that of the control group at 48 h after silencing (Fig. 5F), which was consistent with the findings for Spodoptera frugiperda [52]. The specific mechanism needs further study. Pesch et al. found that the silencing of Cht2 affected the level of chitin in the Drosophila midgut [74]. Zhu found that Cht10 of Plutella xylostella was mainly involved in the process of moulting, pupation, and eclosion of larvae [75]. In this study, the expression of the two chitinase genes Cht2 and Cht10 was significantly or very significantly upregulated at 24 and 48 h after CHS-2 silencing (Fig. 5B). However, the activity of chitinase in the midgut remained unchanged (Fig. 6B). It is speculated that one reason may be the time difference between gene expression and enzyme protein expression, and the other reason is that CHS-2 silencing upregulates CHS expression in other parts of Ae. albopictus larvae aside from the midgut. CHS-2 silencing caused a significant decrease in chitin levels in the larval midgut (Fig. 6A), consistent with other studies [62, 76]. In this study, samples of Ae. albopictus larvae after CHS-2 silencing were collected for paraffin embedding, sectioning, and HE staining, and the results showed that the formation of the peritrophic membrane in the midgut of Ae. albopictus was affected after CHS-2 silencing. The peritrophic membrane was thinner or even absent and the midgut epithelial cells were vacuolated (Fig. 7), which was similar to the results of previous studies [74, 77].
Overall, the results showed that CHS-2 could affect the synthesis and degradation of chitin in the midgut of Ae. albopictus larvae, thus affecting the structure and function of the intestinal peritrophic membrane. The peritrophic membrane is a semipermeable tissue made of chitin and proteins that helps protect insects from pathogens and macromolecular compounds. Although silencing of CHS-2 does not lead directly to larval death, broken peritrophic membranes may make larvae more sensitive to insecticides or heavy metal pollution. In a follow-up study in our laboratory (data not yet published), the mortality of Ae. albopictus larvae was significantly increased in response to cadmium stress after the CHS-2 gene was silenced. Therefore, CHS-2 represents a potential target for auxiliary control of Ae. albopictus, providing a theoretical basis for the population control of Ae. albopictus. Future research will focus on the following: first, delivery of dsRNA, where microinjection is obviously impractical, the exploration of more accessible silencing methods, such as nanocarriers [78], feeding [79], and gene editing [80, 81]. Further studies on the specific effects of CHS-2 silencing on other physiological characteristics of Ae. albopictus, including maturation, reproduction, and stress resistance, are needed to facilitate the development of biocides targeting chitin synthesis and metabolism.
Conclusions
We analysed the expression characteristics of the CHS-2 gene in Ae. albopictus, and found that it was mainly expressed in the midgut, with the highest expression level in adult mosquitoes after blood-feeding. Compared with that in the midgut of larvae in the control group, the level of chitin in the midgut of larvae of the CHS-2 knockdown group was significantly reduced, and the structure of the midgut peritrophic membrane was destroyed or even absent. Our results demonstrate that CHS-2 is important for the formation of the peritrophic membrane in the midgut of Ae. albopictus. CHS-2 gene silencing, although not directly affecting the survival of Ae. albopictus, can still be used to enhance the effects of other control methods, which may lead to a new direction for vector insect control and provide new ideas for research on reducing pest resistance.
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional files. Raw data are available from the corresponding author upon request.
Abbreviations
- CHS :
-
Chitin synthetase gene
- CHS-1 :
-
Chitin synthase-1
- CHS-2 :
-
Chitin synthase-2
- BPU:
-
Benzophenylurea
- qPCR:
-
Real-time fluorescence-based quantitative PCR
- TRE1 :
-
Trehalase 1
- TRE2 :
-
Trehalase 2
- HK:
-
Hexokinase
- G6PI:
-
Glucose-6-phosphate isomerase
- GFAT:
-
Glutamine–fructose-6-phosphate aminotransferase
- GNPNA:
-
Glucosamine-6-phosphate-n-acetyltransferase
- PAGM:
-
Phosphoacetylglucosamine mutase
- UAP:
-
Uridine diphosphate (UDP)-N-acetylglucosamine pyrophosphorylase
- CHS:
-
Chitin synthetase
- CHSA:
-
Chitin synthetase A
- CHSB:
-
Chitin synthetase B
- RNAi:
-
RNA interference
- NCBI:
-
National Center for Biotechnology Information
- GFP :
-
Green fluorescent protein gene
References
Chiodini J. Apps from the World Health Organization – The World Malaria Report and more. Travel Med Infect Dis. 2018;22:82–4.
Chattopadhyay A, Shaw V, Mukherjee P, Ghosh S, Banerjee PK. Development of plant-based larvicide and herbal mosquito repellent fast card with reference to identification of the functional bioactive compounds effective against Culex mosquito. Appl Biochem Biotechnol. 2022;194:2419–30.
Wilke ABB, Wisinski BF, Benelli G, Vasquez C, Mutebi JP, Petrie WD, et al. Local conditions favor dengue transmission in the contiguous United States. Entomol Gen. 2021;41:521–9.
Matsuo N. Discovery and development of pyrethroid insecticides. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95:378–400.
Lopez SBG, Guimarães-Ribeiro V, Rodriguez JVG, Dorand F, Salles TS, Sá-Guimarães TE, et al. RNAi-based bioinsecticide for Aedes mosquito control. Sci Rep. 2019;9:4038.
Naqqash MN, Gökçe A, Bakhsh A, Salim M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol Res. 2016;115:1363–73.
Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96.
Seo SM, Lee JW, Shin J, Tak JH, Hyun J, Park IK. Development of cellulose nanocrystal-stabilized Pickering emulsions of massoia and nutmeg essential oils for the control of Aedes albopictus. Sci Rep. 2021;11:12038.
Pavela R, Maggi F, Iannarelli R, Benelli G. Plant extracts for develo** mosquito larvicides: from laboratory to the field, with insights on the modes of action. Acta Trop. 2019;193:236–71.
Quinn C, Anthousi A, Wondji C, Nolan T. CRISPR-mediated knock-in of transgenes into the malaria vector Anopheles funestus. G3. 2021;11:jkab201.
Caragata EP, Dong S, Dong Y, Simoes ML, Tikhe CV, Dimopoulos G. Prospects and pitfalls: next-generation tools to control mosquito-transmitted disease. Annu Rev Microbiol. 2020;74:455–75.
Dong S, Dong Y, Simoes ML, Dimopoulos G. Mosquito transgenesis for malaria control. Trends Parasitol. 2022;38:54–66.
Yooyangket T, Muangpat P, Polseela R, Tandhavanant S, Thanwisai A, Vitta A. Identification of entomopathogenic nematodes and symbiotic bacteria from Nam Nao National Park in Thailand and larvicidal activity of symbiotic bacteria against Aedes aegypti and Aedes albopictus. PLoS ONE. 2018;13:e0195681.
Kajla MK. Symbiotic bacteria as potential agents for mosquito control. Trends Parasitol. 2020;36:4–7.
Spindler KD, Spindler-Barth M, Londershausen M. Chitin metabolism: a target for drugs against parasites. Parasitol Res. 1990;76:283–8.
Zhu KY, Merzendorfer H, Zhang W, Zhang J, Muthukrishnan S. Biosynthesis, turnover, and functions of chitin in insects. Annu Rev Entomol. 2016;61:177–96.
Merzendorfer H. Insect chitin synthases: a review. J Comp Physiol B. 2006;176:1–15.
Konno K, Mitsuhashi W. The peritrophic membrane as a target of proteins that play important roles in plant defense and microbial attack. J Insect Physiol. 2019;117:103912.
Erlandson MA, Toprak U, Hegedus DD. Role of the peritrophic matrix in insect-pathogen interactions. J Insect Physiol. 2019;117:103894.
Wu H, Zhao D, Guo XC, Liu ZR, Li RJ, Lu XJ, et al. Group V chitin deacetylases influence the structure and composition of the midgut of Beet armyworm, Spodoptera exigua. Int J Mol Sci. 2023;24:3076.
Liu D, De Schutter K, Far J, Staes A, Dewettinck K, Quinton L, et al. RNAi of Mannosidase-Ia in the Colorado potato beetle and changes in the midgut and peritrophic membrane. Pest Manag Sci. 2022;78:5071–9.
Stalin A, Daniel Reegan A, Rajiv Gandhi M, Saravanan RR, Balakrishna K, Hesham AE, et al. Mosquitocidal efficacy of embelin and its derivatives against Aedes aegypti L. and Culex quinquefasciatus Say. (Diptera: Culicidae) and computational analysis of acetylcholinesterase 1 (AChE1) inhibition. Comput Biol Med. 2022;146:105535.
Marco K, Jothini O-N, Subbaratnam M, Hans M. Chitin is a necessary component to maintain the barrier function of the peritrophic matrix in the insect midgut. Insect Biochem Molec. 2015;56:21–8.
Kaya M, Sofi K, Sargin I, Mujtaba M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohyd Polym. 2016;145:64–70.
Guillaume T, ** W. Chitinous structures as potential targets for insect pest control. Adv Exp Med Biol. 2019;1142:273–92.
Luo YJ, Chen Y, Wang XJ, Wang ST, Yang YY, Xu HX, et al. Validamycin affects the development and chitin metabolism in Spodoptera frugiperda by inhibiting trehalase activity. Entomol Gen. 2022;42:931–9.
Tian X, Zhang C, Xu Q, Li Z, Shao X. Azobenzene-benzoylphenylureas as photoswitchable chitin synthesis inhibitors. Org Biomol Chem. 2017;15:3320–3.
Cohen E. Chitin synthesis and inhibition: a revisit. Pest Manag Sci. 2001;57:946–50.
Xu CD, Liu YK, Qiu LY, Wang SS, Pan BY, Li Y, et al. GFAT and PFK genes show contrasting regulation of chitin metabolism in Nilaparvata lugens. Sci Rep. 2021;11:5246.
Merzendorfer H. The cellular basis of chitin synthesis in fungi and insects: common principles and differences. Eur J Cell Biol. 2011;90:759–69.
Au-Young J, Robbins PW. Isolation of a chitin synthase gene (CHS1) from Candida albicans by expression in Saccharomyces cerevisiae. Mol Microbiol. 1990;4:197–207.
Tellam RL, Eisemann C. Chitin is only a minor component of the peritrophic matrix from larvae of Lucilia cuprina. Insect Biochem Molec. 2000;30:1189–201.
Li L, Wang YQ, Li GY, Song QS, Stanley D, Wei SJ, et al. Genomic and transcriptomic analyses of chitin metabolism enzymes in Tenebrio molitor. Arch Insect Biochem Physiol. 2022;111:e21950.
Merzendorfer H, Zimoch L. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol. 2003;206:4393–412.
Gagou ME, Kapsetaki M, Turberg A, Kafetzopoulos D. Stage-specific expression of the chitin synthase DmeChSA and DmeChSB genes during the onset of Drosophila metamorphosis. Insect Biochem Mol Biol. 2002;32:141–6.
Arakane Y, Specht CA, Kramer KJ, Muthukrishnan S, Beeman RW. Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 2008;38:959–62.
Kumar NS, Tang B, Chen X, Tian H, Zhang W. Molecular cloning, expression pattern and comparative analysis of chitin synthase gene B in Spodoptera exigua. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:447–53.
Zhang X, Zhang J, Park Y, Zhu KY. Identification and characterization of two chitin synthase genes in African malaria mosquito, Anopheles gambiae. Insect Biochem Mol Biol. 2012;42:674–82.
Shi JF, Mu LL, Chen X, Guo WC, Li GQ. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). Int J Biol Sci. 2016;12:1319–31.
Zeng B, Chen FR, Sun H, Liu Y, Wu SF, Bass C, et al. Molecular and functional analysis of chitin synthase genes in Chilo suppressalis (Lepidoptera: Crambidae). Insect Sci. 2022;30:1–16.
Zhang X, Zhang J, Zhu KY. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol Biol. 2010;19:683–93.
Yang XS, Xu Y, Yin Q, Zhang HB, Yin HT, Sun Y, et al. Physiological characterization of chitin synthase A responsible for the biosynthesis of cuticle chitin in Culex pipiens pallens (Diptera: Culicidae). Parasit Vectors. 2021;14:234.
Guo JX, Xu Y, Yang XS, Sun XH, Sun Y, Zhou D, et al. TRE1 and CHS1 contribute to deltamethrin resistance in Culex pipiens pallens. Arch Insect Biochem Physiol. 2019;100:e21538.
Lu ZJ, Huang YL, Yu HZ, Li NY, **e YX, Zhang Q, et al. Silencing of the chitin synthase gene Is lethal to the Asian Citrus psyllid, Diaphorina citri. Int J Mol Sci. 2019;20:3734.
Zhang JZ, Liu XJ, Zhang JQ, Li DQ, Sun Y, Guo YP, et al. Silencing of two alternative splicing-derived mRNA variants of chitin synthase 1 gene by RNAi is lethal to the oriental migratory locust, Locusta migratoria manilensis (Meyen). Insect Biochem Mol Biol. 2010;40:824–33.
Mohammed AMA, Diab MR, Abdelsattar M, Khalil SMS. Characterization and RNAi-mediated knockdown of Chitin synthase A in the potato tuber moth, Phthorimaea operculella. Sci Rep. 2017;7:9502.
Palatini U, Masri RA, Cosme LV, Koren S, Thibaud-Nissen F, Biedler JK, et al. Improved reference genome of the arboviral vector Aedes albopictus. Genome Biol. 2020;21:215.
Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7:76–85.
Hsu PC, Atlihan R, Chi H, Dai SM. Comparative demography and mass rearing of Aedes aegypti fed on different food sources using a novel perforated feeder. Entomol Gen. 2022;42:827–34.
Kaur G, Iyer LM, Subramanian S, Aravind L. Evolutionary convergence and divergence in archaeal chromosomal proteins and chromo-like domains from bacteria and eukaryotes. Sci Rep. 2018;8:6196.
Avicor SW, Wajidi MF, Jaal Z, Yahaya ZS. Molecular cloning, sequence analysis and developmental stage expression of a putative septin gene fragment from Aedes albopictus (Diptera: Culicidae). Acta Biochim Pol. 2016;63:243–6.
Liu XY, Wang SS, Yu YY, Cheng YS, Hu CX, Zhou M, et al. Effects of inhibiting the expression of chitin synthase gene sfCHSB on the metabolism of trehalose and chitin in Spodoptera frugiperda larvae. Agriculture. 2022;12:2019.
Gregor KM, Becker SC, Hellhammer F, Schon K, Baumgartner W, Puff C. Histochemical staining techniques in Culex pipiens and Drosophila melanogaster (Diptera) with a comparison to mammals. Vet Pathol. 2022;59:836–49.
Tellam RL, Vuocolo T, Johnson SE, Jarmey J, Pearson RD. Insect chitin synthase cDNA sequence, gene organization and expression. Eur J Biochem. 2000;267:6025–43.
Yu HZ, Li NY, **e YX, Zhang Q, Wang Y, Lu ZJ. Identification and functional analysis of two chitin synthase genes in the common cutworm, Spodoptera litura. Insects. 2020;11:253.
Zhu YC, Specht CA, Dittmer NT, Muthukrishnan S, Kanost MR, Kramer KJ. Sequence of a cDNA and expression of the gene encoding a putative epidermal chitin synthase of Manduca sexta. Insect Biochem Mol Biol. 2002;32:1497–506.
Zimoch L, Hogenkamp DG, Kramer KJ, Muthukrishnan S, Merzendorfer H. Regulation of chitin synthesis in the larval midgut of Manduca sexta. Insect Biochem Mol Biol. 2005;35:515–27.
Zhao YJ, Sui XY, Xu LJ, Liu GY, Lu LH, You MS, et al. Plant-mediated RNAi of grain aphid CHS1 gene confers common wheat resistance against aphids. Pest Manag Sci. 2018;74:2754–60.
Ullah F, Gul H, Wang X, Ding Q, Said F, Gao X, et al. RNAi-mediated knockdown of chitin synthase 1 (CHS1) gene causes mortality and decreased longevity and fecundity in Aphis gossypii. Insects. 2019;11:22.
Khalil SMS, Munawar K, Alahmed AM, Mohammed AMA. RNAi-mediated screening of selected target genes against Culex quinquefasciatus (Diptera: Culicidae). J Med Entomol. 2021;58:2177–85.
Rana S, Rajurkar AB, Kumar KK, Mohankumar S. Comparative analysis of chitin synthase A dsRNA mediated RNA interference for management of crop pests of different families of Lepidoptera. Front Plant Sci. 2020;11:427.
Singh AD, Wong S, Ryan CP, Whyard S. Oral delivery of double-stranded RNA in larvae of the Yellow fever mosquito, Aedes aegypti: implications for pest mosquito control. J Insect Sci. 2013;13:1–18.
Zhao W, Zhang C, Zhai S, Sun Q, Zhang J. Expression and analysis of chitin synthase genes cqCHS1 and cqCHS2 in Culex drowsiness mosquitoes. Genomics Appl Biol. 2016;35:2317–23.
Chen L, Yang WJ, Cong L, Xu KK, Wang JJ. Molecular cloning, characterization and mRNA expression of a chitin synthase 2 gene from the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Int J Mol Sci. 2013;14:17055–72.
Ibrahim GH, Smartt CT, Kiley LM, Christensen BM. Cloning and characterization of a chitin synthase cDNA from the mosquito Aedes aegypti. Insect Biochem Mol Biol. 2000;30:1213–22.
Zhuo W, Chu F, Kong L, Tao H, Sima Y, Xu S. Chitin synthase B: a midgut-specific gene induced by insect hormones and involved in food intake in Bombyx mori larvae. Arch Insect Biochem Physiol. 2014;85:36–47.
Moreira MF, Dos Santos AS, Marotta HR, Mansur JF, Ramos IB, Machado EA, et al. A chitin-like component in Aedes aegypti eggshells, eggs and ovaries. Insect Biochem Mol Biol. 2007;37:1249–61. GH, MN, CR, KJ, S
Zhang Y, Feng ZJ, Chen ZS, Wang XX, Cong HS, Fan YL, et al. Connection between cuticular hydrocarbons and melanization in Harmonia axyridis revealed by RNAi-mediated silencing of the CYP4G79. Entomol Gen. 2020;41:83–96.
Ullah F, Gul H, Tariq K, Hafeez M, Desneux N, Gao XW, et al. RNA interference-mediated silencing of ecdysone receptor (EcR) gene causes lethal and sublethal effects on melon aphid. Aphis gossypii Entomol Gen. 2022;42:791–7.
Yan S, Yin MZ, Shen J. Nanoparticle-based nontransformative RNA insecticides for sustainable pest control: mechanisms, current status and challenges. Entomol Gen. 2022;43:21–30.
Yu HZ, Huang YL, Lu ZJ, Zhang Q, Su HN, Du YM, et al. Inhibition of trehalase affects the trehalose and chitin metabolism pathways in Diaphorina citri (Hemiptera: Psyllidae). Insect Sci. 2021;28:718–34.
Pan BY, Li GY, Wu Y, Zhou ZS, Zhou M, Li C. Glucose utilization in the regulation of chitin synthesis in Brown planthopper. J Insect Sci. 2019;19:3.
Yang WJ, Wu YB, Chen L, Xu KK, **e YF, Wang JJ. Two chitin biosynthesis pathway genes in Bactrocera dorsalis (Diptera: Tephritidae): molecular characteristics, expression patterns, and roles in larval-pupal transition. J Econ Entomol. 2015;108:2433–42.
Pesch YY, Riedel D, Behr M. Drosophila chitinase 2 is expressed in chitin producing organs for cuticle formation. Arthropod Struct Dev. 2016;46:4–12.
Zhu B, Shan JQ, Li R, Liang P, Gao XW. Identification and RNAi-based function analysis of chitinase family genes in diamondback moth, Plutella xylostella. Pest Manag Sci. 2019;75:1951–61.
Arakane Y, Hogenkamp DG, Zhu YC, Kramer KJ, Specht CA, Beeman RW, et al. Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem Mol Biol. 2004;34:219–304.
Liu XJ, Zhang HH, Li S, Zhu KY, Ma EB, Zhang JZ. Characterization of a midgut-specific chitin synthase gene (LmCHS2) responsible for biosynthesis of chitin of peritrophic matrix in Locusta migratoria. Insect Biochem Mol Biol. 2012;42:902–10.
Yang W, Wang B, Lei G, Chen G, Liu D. Advances in nanocarriers to improve the stability of dsRNA in the environment. Front Bioeng Biotechnol. 2022;10:974646.
Kunte N, McGraw E, Bell S, Held D, Avila LA. Prospects, challenges and current status of RNAi through insect feeding. Pest Manag Sci. 2020;76:26–41.
Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2016;34:78–83.
Choo A, Fung E, Nguyen TNM, Okada A, Crisp P. CRISPR/Cas9 mutagenesis to generate novel traits in Bactrocera tryoni for sterile insect technique. Methods Mol Biol. 2022;2495:151–71.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China [Grant Numbers 32272608 and 31672081].
Author information
Authors and Affiliations
Contributions
YJD and SGW designed the study, and YJD, CZ, MZ, and YT performed the research. CZ, RFC, YTW, and YRC analysed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal procedures have been approved by the Experimental Animal Ethics Committee (AEWC) of Hangzhou Normal University (Approval No. HSD20220103).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, C., Ding, Y., Zhou, M. et al. RNAi-mediated CHS-2 silencing affects the synthesis of chitin and the formation of the peritrophic membrane in the midgut of Aedes albopictus larvae. Parasites Vectors 16, 259 (2023). https://doi.org/10.1186/s13071-023-05865-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05865-3